Introduction
In the dynamic landscape of reproductive health, combined oral hormonal contraception (COC) continues to be a pivotal area of research and development. This paper provides a timely and comprehensive overview of well-known facts and recent advancements in COC, aiming to summarize the latest findings, address challenges and highlight potential pitfalls.
Estrogens in oral combined hormonal contraception
In contemporary formulations, the estrogen component within COCs is dispensable for the inhibition of ovulation, as the progestin component alone is adequate to achieve this effect. The inclusion of the estrogen component aims primarily at minimizing breakthrough bleeding during COC application. Estrogens are the main reason for vascular complications, such as venous thromboembolism (VTE) in women.
Ethinylestradiol (EE) stands out as the most widely used estrogen in COCs. The introduction of the ethinyl-group in EE results in a delayed metabolism in both the liver and the endometrium, thereby fostering a more consistent and stable bleeding pattern [1]. COCs containing EE and estradiol valerate (E2V) and estradiol (E2) have been accessible in the European Union for more than a decade.
EE, E2 and E2V induce the synthesis of clotting factors as well as fibrinolytic factors in the liver, causing imbalance in the coagulation system. This results in an elevated risk for VTE, especially in the early phase of use. In comparison to E2, EE shows a lower first pass effect in the liver due to the 17-alpha-ethinyl group which prevents the inactivation of the molecule, leading to a longer half-life. In consequence there is a stronger impact on hepatic metabolism for EE. Non-oral administration of EE in combination with a progestin for contraception was not associated with a significantly lower impact on the clotting system, binding proteins or plasma lipids. This is in accordance with studies demonstrating no risk difference for VTE in combined hormonal contraception (CHC) using vaginal, transdermal or oral administration [2,3]. Although combinations with E2 demonstrate a slightly lower impact on some clotting parameters and binding globulins, the clinical outcome related to VTE risk does not differ.
While there is no negative impact of EE on plasma lipids, it induces an increased production of binding globulins. The high levels of sex hormone binding globulin (SHBG) reduce the plasma levels of free testosterone. Lower free testosterone results in a positive impact on the skin and reduction in acne. There is a considerable interindividual variability in steroid metabolism, applicable to both estrogens and progestins. Hence, it is not unexpected that a uniform dose may induce side effects in one individual while the same substance is very well-tolerated by other women. Pharmacological studies have demonstrated a 74% increase in SHBG levels with the administration of EE/Levonorgestrel (LNG) and a 215% increase in SHBG levels with EE/drospirenone (DRSP). Another publication indicated a comparable effect on SHBG between EE/LNG and E2V/dienogest (DNG), with no significant differences observed [4-7].
Estetrol (E4) is a relatively weak estrogen, produced in the fetal liver and detectable in humans exclusively during pregnancy [8]. Klipping et al. [7] showed that the administration of E4/DRSP has a lower impact on endocrine and metabolic parameters. The influence on gonadotropins, cortisol, angiotensinogen, and triglycerides was less prominent when compared with products containing EE. In contrast to EE/DRSP, EE/LNG and E2V/DNG, the rise in SHBG levels with E4/DRSP intake was comparatively moderate with a 55% increase [7]. The first preparation pill using E4, as the estrogenic component, containing 14.2 mg E4 and 3 mg DRSP in a monophasic 24/4 regimen was approved in Europe in 2022. Investigations by Gemzell et al. in Europe and Russia, as well as Creinin et al. in the USA, have demonstrated a high contraceptive efficacy (Pearl Index 0.47 in the European study). Data also indicate slightly lower metabolic effects on hemostasis parameters in the liver compared to EE/LNG and EE/DRSP [9]. However, whether this translates in a lower incidence of VTE compared to pills with other estrogens requires further follow-up in Phase 4 studies.
Progestins in oral combined hormonal contraception
Various types of progestins exist in COCs, resulting in a vast variety of available pills on the market. Classification can be based on the chemical origin of the progestin components, their generation, or their physiological effects.
From a chemical perspective, progestins are categorized into 17alpha-hydroxyprogesterone derivatives (e.g. cyproterone acetate, chlormadinone acetate or medroxyprogesterone acetate), 19-nortestosterone derivatives classified as estranes (e.g. norethisterone acetate or DNG) or gonanes (e.g. LNG, desogestrel, or gestodene), and spironolactone derivatives such as DRSP.
LNG and norethisterone are second-generation progestins and gestodene and desogestrel are third generation progestins. The spironolactone derivative DRSP is classified as a fourth-generation progestin.
Progestins as well as estrogens are steroid hormones that may bind to other steroid hormone receptors and exert there an agonistic or antagonistic effect. Progesterone itself exerts little anti-androgenic and anti-mineralocorticoid effects. The synthetic progestins LNG, gestodene, and desogestrel exert small androgenic effects, while DRSP, DNG, cyproterone acetate, and chlormadinone acetate display anti-androgenic effects [10,11]. Anti-mineralocorticoid effects are observed with DRSP due to its structural similarity to spironolactone [12].
Bleeding pattern
The expected bleeding pattern differs between different COCs and progestin-only pills (POP). Under treatment with POP more frequent, longer bleeding episodes occur in non-predictable intervals [13]. Within the group of COCs, unscheduled bleeding often occurs during the first cycles of use and decreases over time. COCs containing E2 and E4 have a less stable bleeding pattern with more unscheduled bleeding episodes and absence of withdrawal bleedings in up to 20% of the cycles. A combination of E4/DRSP causes more breakthrough bleeding when compared to other COCs with 15.5-19.2% of unscheduled bleeding episodes after cycle 4. Unscheduled bleeding episodes and absence of withdrawal bleeding decrease from cycle 3 to cycle 11 (12.8% and 13% at the 11th cycle) [14,15]. Adequate patient counselling before prescription is crucial to inform about the harmlessness of these bleedings and impede discontinuation. Regarding E2V the dynamic dosing regimen of E2V/DNG shows a comparable cycle control to EE/LNG. While E2V/DNG shows an equal amount of bleeding episodes when compared to EE/LNG, the episodes seem to be shorter, while absence of withdrawal bleeding is also more frequent in the E2V/DNG group [16].
Cardiovascular risk assessment
Use of COCs is associated with a higher risk for cardiovascular events, especially in women with certain risk factors. It is crucial to distinguish between the risk of VTE and arterial events. VTE mostly stem from an acute event arising from the pro-coagulatory metabolic effects of hormonal contraceptives. These complications typically impact younger users and new users. In contrast, arterial events typically occur when arteries are already compromised by atherosclerosis, with thrombus formation on the plaques potentially promoted by the effects of COCs on coagulation. Noteworthy risk factors for arterial events include age greater than 35 years, smoking, hypertension, diabetes and other conditions associated with atherosclerosis. The use of COCs is associated with an increased risk for both types of events [17-21].
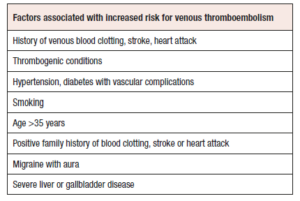
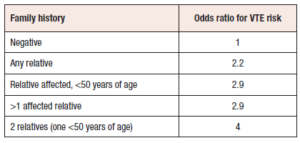
Careful assessment of the patient's general medical history is imperative to identify key aspects and mitigate the risk of severe complications. Previous incidents of venous blood clots, strokes, or heart attacks should be ruled out. Additional risk factors are presented in Table 1 and encompass VTE in the personal history, thrombogenic mutations, migraine with aura, hypertension (systolic ≥160/diastolic ≥100 mmHg), severe liver or gallbladder disease, systemic lupus erythematosus, diabetes with vascular complications, smoking beyond the age of 35 years, or a positive family history of blood clotting, stroke, or heart attack in any first-degree relative under the age of 50 years [20].Table 2 illustrates the relative risk increase in for VTE based on both the number of affected family members as well as their age at onset of VTE [22-28].
Risk for venous thromboembolism
The risk for VTE in healthy COC users is very low. The absolute annual risk for VTE in healthy young women is only 2-3 per 10,000 women-years and is 2-4 fold in COC users (5-12/10,000 women-years). The VTE risk during a pregnancy would be much higher with 58-60 events per 10,000 women-years [20]. The risk factors shown in Table 1 do not merely sum up but cause a multiplicative risk increase.
VTE risk varies depending on the combination of estrogen and progestin components. The combinations with the smallest increased risk, and therefore the safest options, are EE combined with second generation progestins (like LNG, norethisterone) as well as a combination of E2 and nomegestrol acetate (NOMAC) with a risk for VTE of 4.8-8.9 per 10,000 women-years [18,19,21,29].
EE combined with the third generation progestin LNG is associated with between 3 and 6.9 VTE per 10,000 women-year [19,30], while EE in combination with third generation progestins or DRSP lead to a VTE risk of 9-12 per 10,000 women-years [21]. E2V combined with DNG is associated with a VTE risk of 6.9 per 10,000 women-years [18]. The Reed study was powered to compare the VTE risk in E2/NOMAC users compared to EE/LNG users (2 per 10,000 women-years and 3 per 10,000 women-years, respectively) [19]. No difference between the two preparations was found. Insofar both are considered at present as the combined pills with the lowest risk for VTE. The absolute risk for COCs containing E4 and DRSP is not yet known. No significant difference in VTE risk was found between pills containing 20 mcg or 30 mcg EE [31]. Overall COCs containing EE in combination with a third or fourth-generation progestin exhibit a twofold higher risk of VTE when compared with COCs incorporating EE with a second-generation progestin or E2/NOMAC.
In conclusion, it is advisable to initiate COC with products with the lowest risk for VTE. At present these are COC containing EE and LNG or norgestimate and E2/NOMAC if there is no specific medical indication requiring a COC with a hormonal combination associated with a slightly higher risk of VTE [19]. Furthermore, annual reassessment of risk factors is imperative to ensure ongoing safety in contraceptive choices.
Risk of breast cancer and COC use
In line with older studies, more recent studies by Mørch et al. indicate an elevated risk of breast cancer in COC users, with an additional 13 cases of breast cancer per 100,000 women-years [31,32]. Overall, data suggests a low risk but a continuous increase with the duration of use, with a relative risk of 1.46 for a duration of more than 10 years compared to 1.17 for a duration of 1 to <5 years [31,33]. Whether the risk might decrease after discontinuation of use remains unclear.
Current evidence suggests that the increased risk of breast cancer associated with the use of COCs may also depend on the type and dosage of the used progestins, since data also indicate an increased breast cancer risk with POPs and the LNG-releasing intrauterine device (LNG-IUD) [34,35]. Additionally, the Mørch study suggests a potential influence of the progestin component related to the higher risk for COCs. However, there are currently no conclusive data regarding the breast cancer risk in different combinations of COC [32]. Older data that demonstrated differences are not conclusive due to the higher doses of estrogen components in COCs at that time compared to today, as well as the limited number of users of COCs containing third-generation progestins [36].
Breast cancer risk and COC use in women BRCA1/BRCA2 mutation carriers
Contraceptive counselling is complex for carriers of BRCA1 and BRCA2 mutations. Carriers of these mutations already have a significantly increased baseline risk for breast cancer (54-75% for BRCA1-mutation carriers and 45% for BRCA2-mutation carriers) and ovarian cancer (18-60% for BRCA1-mutation carriers and 11-27% for BRCA2-mutation carriers) [37-41].
BRCA1 and COC use
Kotsopoulos et al. found an odds ratio (OR) of 1.18 for COC ever use compared to never users in BRCA1 carriers. The risk increase was higher with a duration of use exceeding 5 years (OR 1.22) or first use under 20 years of age (OR 1.45). The risk for a breast cancer diagnosis before the age of 40 years was also increased with an OR of 1.38 [42].
Retrospective analyses have shown a significantly increased breast cancer risk with duration of use of more than 10 years. Another set of retrospective data showed a significant association between younger age at COC initiation and risk of breast cancer [43].
In contrast, a meta-analysis performed in 2018 using prospective data showed no significant increase in breast cancer with the age at first use and the duration of use for women with BRCA1 mutation using COC. These inconsistent findings are interpreted by the authors as related to the inadequate study design with low representation of younger women in the prospective cohort and survival bias [43]. Altogether based on present knowledge non-hormonal contraceptive methods are the method of choice in women with BRCA1.
BRCA2 and CHC use
For women with BRCA2 mutations, Haile et al. found no significant association between the risk for breast cancer and the use of COCs for one year, however a strong increase of risk for breast cancer was found in women who used COCs for more than 5 years (OR 2.0) [44]. Likewise, Brohet et al. found a significant risk increase with COC use, most significantly with a duration of use of 4-8 years with an OR of 2.3 compared to non-users [45]. Similar to BRCA1, the 2018 meta-analysis by Schrijver et al. with several limitations found no risk increase in prospective data possibly due to survival bias or the low number of young women in the cohort [43]. Retrospective data again showed a risk increase for women of younger age at the start of COC use (18-22 years) and longer duration of use (>10 years) [43].
In summary, high quality studies demonstrate a significant further increase of breast cancer risk in women using COCs carrying BRCA1 and BRCA2 mutations. Certain circumstances such as a younger age at the start of COC use or longer duration of use might lead to an even higher elevation of the risk. On the other hand, it is well known that COCs decrease the risk for ovarian cancer in this high-risk population. Moorman et al. showed a significant risk reduction for ovarian cancer in BRCA1 and 2 mutation carriers with COC use with an OR of 0.58 and 0.46 to 0.73 respectively [46]. This effect seems to accumulate with an increasing duration of COC use and might potentially persist long after cessation of use, at least for BRCA 1 mutation carriers [47,48]. However, as today prophylactic salpingo-oophorectomy is the method of choice to avoid ovarian cancer in this population, it cannot be ethically justified to increase the already high risk for breast cancer alone with the aim to reduce the risk for ovarian cancer. A qualified risk-benefit discussion has to be conducted with the patient which has to include all reproductive aims [49,50]. A recent work by Kotsopoulos et al. additionally showed a significant reduction of all-cause mortality at 75 years of age for female BRCA1/2 carriers who had an oophorectomy at the age of 35 compared to patients without oophorectomy, with an all-cause mortality of 25% compared to 62% for female BRCA1 carriers and 12% compared to 28% for female BRCA2 carriers, highlighting the importance of prophylactic oophorectomy in this population [51].
COC use in special situations
Non-contraceptive health benefits
Migraine
The prevalence of migraine among females has been determined to be 13.8%, with 50% of these cases exhibiting an association with the menstrual cycle [52-55]. Estrogen withdrawal seems to play a pivotal role here. Notably, statistical data establishes a significant correlation between migraines and ischemic stroke, with an OR ranging from 2.3 to 3.8 for migraine without aura and 3.8 to 8.6 for migraine with aura, respectively, when compared to healthy women. This risk for stroke increases significant with the use of COCs (OR ranging from 13.9 to 16.9). Among smokers with migraine using COC, the OR for such an event is even higher (OR 34.4) [56,57].
Moreover, the administration of COCs in susceptible individuals can serve as a triggering factor for migraines, exacerbating of existing migraine, or can initiate auras in women with non-aura migraines [58]. These scenarios require an immediate discontinuation of COC use, highlighting the importance of clinical vigilance in managing these interconnected health considerations.
Therefore, a comprehensive medical history is imperative, encompassing details regarding the onset and frequency of migraine, aura, intensity, response to pain medication and family history. Data suggesting an association between the occurrence and severity of symptoms during migraine, depression, and endometriosis underscores the importance of additionally placing particular emphasis on these comorbidities in the medical history [59,60]. While migraine without aura does not constitute an absolute contraindication to start contraception with COC, it is essential to consider that the frequency of migraines may escalate with COC usage. In such cases, a POP or intrauterine copper-device could be the better option. The POP containing 75 µg desogestrel may even be used as a therapy for migraine since has been shown to reduce the frequency of headaches, the intensity of headaches and the use of triptans which lead to a significant improvement in the quality of life [11,61].
Heavy menstrual bleeding and acne
Other non-contraceptive health benefits include the treatment of acne and heavy menstrual bleedings. In the case of heavy menstrual bleedings, a treatment with COCs shows to be equally effective as treatment with tranexamic acid. This benefit was even more significant for patients with leiomyomas [63]. When it comes to acne there is a significant reduction of symptoms during the use of COCs especially in preparations with antiandrogenic progestins. EE-induced SHBG reduction also contributes to acne improvement.
COC use after emergency contraception
If COCs are prescribed in the context of an initial consultation for the purpose of postcoital emergency contraception with ulipristal acetate (UPA), careful consideration of interactions between UPA and COCs is crucial. In a prospective cohort study conducted by Edelman et al., the administration of COCs within a two-day window following the intake of UPA was found to lead to a substantial increase in the incidence of follicle ruptures within a five-day period post-administration of UPA. This observed phenomenon subsequently leads to a significant reduction in the efficacy of UPA as an emergency contraceptive method [63].
The initiation of COC intake should occur no earlier than 5 days after UPA administration. It is advisable to use condoms as a contraceptive method until the onset of the next menstrual bleeding, followed by the commencement of the COC on the first day of bleeding. This approach optimizes the effectiveness of the UPA emergency contraception.
Summary
In summary, to support the patient in the choice of a contraceptive method and to reduce the occurrence of adverse events for the individual patient a huge variety of factors needs to be taken into consideration. Every consultation should include the patient's individual history, encompassing both risk factors and lifestyle preferences, such as the desire for monthly bleeding or amenorrhea, financial situation, personal preference, compliance or adherence. Whenever possible, the initial choice should prioritize COCs with the lowest risk for VTE, such as EE/LNG or E2/NOMAC.
Conflict of interest statement
The authors declare having no conflicts of interest.