Introduction
Vasomotor symptoms such as hot flushes and night sweats are often considered the cardinal symptoms of menopause and have a substantial detrimental effect on women’s health-related quality of life [1-6]. The high proportion of women who experience perimenopausal vasomotor symptoms worldwide highlights the need for effective, well-tolerated treatments. Estrogen-based menopausal hormone therapy is the foundation of treatment for estrogen deficiency symptoms, including vasomotor symptoms, and has been shown to reduce symptom frequency and severity and improve quality of life [7-9]. Guidelines recommend tailoring menopausal hormone therapy to individual patients’ symptoms and circumstances using the most appropriate dose of estrogen and, in some cases, selecting the most suitable route of estrogen administration [7,9-14].
For non-hysterectomized women, estrogen is given in combination with progestogen in either cyclic or continuous form [7,10,11]. Low-dose estradiol plus dydrogesterone (1 mg 17β-estradiol combined with 5 mg dydrogesterone; referred hereafter as E 1 mg/D 5 mg) was approved in 2000 for the treatment of estrogen deficiency symptoms and prevention of osteoporosis in postmenopausal women at least 12 months since last menses [12]. Subsequently, ultra-low dose estradiol plus dydrogesterone (0.5 mg 17β-estradiol continuously combined with 2.5 mg dydrogesterone; referred hereafter as E 0.5 mg/D 2.5 mg) was approved in 2010 for the treatment of estrogen deficiency symptoms in postmenopausal women at least 12 months since last menses [12]. Femoston® is the only single fixed dose menopause hormone therapy containing dydrogesterone. Dydrogesterone, like the micronized progesterone, comes from a natural source (e.g. yam and soy) [15,16]. Its unique molecular features are obtained through a photochemical process [17], creating a bent molecular conformation, conferring a high selectivity for progesterone receptors [18,19], with better oral bioavailability compared to oral micronized progesterone [19-21]. Dydrogesterone presents a biological progestogenic, anti-estrogenic, anti-androgenic and anti-mineralocorticoid activity with no anti-gonadotrophic, estrogenic, androgenic, or glucocorticoid actions [19]. Unlike other progestogens, dydrogesterone is non-thermogenetic, non-sedative, does not inhibit gonadotropin release or ovulation, does not influence central nervous system functions and psyche, and does not antagonize various central effects of estrogens [22]. In several randomized, placebo-controlled clinical studies, the ultra-low dose formulations have shown to reduce vasomotor symptoms, including hot flushes and moderate-to-severe hot flushes, in postmenopausal women compared to placebo, with no safety concerns [23-26]. Additionally, patient-reported improvements have also been shown with ultra-low estradiol plus dydrogesterone based on improved scores on the Menopause Rating Scale (MRS) [25,26], a patient-reported outcome (PRO) tool that measures the severity of a range of menopause-related symptoms in aging women with high reliability and validity [27]. PRO tools are increasingly being recognized by regulators and healthcare professionals as valuable in collecting unique, patient-centered information regarding the impact of health conditions and treatments [28]. As such, a closer examination of the impact of low and ultra-low estradiol plus dydrogesterone on MRS scores is warranted. This pooled analysis of data from two phase 3 clinical trials assessed the impact of low and ultra-low estradiol plus dydrogesterone versus placebo on MRS scores assessing symptoms and treatment-related outcomes in postmenopausal women.
Methods
Study design
This pooled analysis included data from two phase 3, double-blind, randomized, placebo-controlled studies that assessed the efficacy of continuous combined oral low and ultra-low dose E and D for the treatment of vasomotor symptoms in postmenopausal women. The first study was conducted in France, Poland, Romania and Russia and the second was conducted in China. Full details of these studies have been reported previously [25,26]. In brief, patients enrolled in the European study were randomized 2:1:2 to receive oral continuous E 0.5 mg/D 2.5 mg, E1 mg/D5 mg or placebo for 13 weeks. Women enrolled in the Chinese study were randomized 1:1 to receive continuous combined E 0.5 mg/D 2.5mg or placebo once daily for 12 weeks [25]. Data from women included in the full analysis samples for the two studies who had received either E 0.5 mg/D 2.5 mg, E1 mg/D5 mg or placebo were included in this pooled analysis.
Both studies were conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice, applicable national/regional regulations and the Declaration of Helsinki and were approved by local ethics committees. All study participants provided written informed consent.
Study population
The European study enrolled non-hysterectomized, postmenopausal women (aged 45–65 years) who had been amenorrhoeic for ≥12 months, had serum estradiol and follicle-stimulating hormone levels within the postmenopausal range, and had ≥ 50 moderate-to-severe hot flushes during the week preceding the start of study treatment. The Chinese study enrolled non-hysterectomized, postmenopausal women (aged 45–60 years) who had been amenorrhoeic for ≥12 months, had serum estradiol <35 pg/mL, follicle-stimulating hormone levels >40 IU/L, and had ≥50 hot flushes over 7 consecutive days during the 2-week screening period. Full eligibility criteria have been published previously [25,26].
Assessments
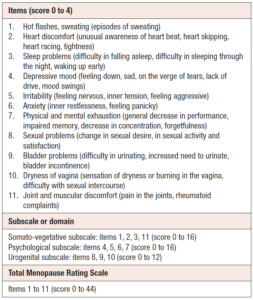
Primary and secondary endpoints for the two studies have been described previously [25,26]. Change in the Menopause Rating Scale (MRS) score from baseline to week 4 and end of treatment (EOT) was assessed in this pooled analysis. During the assessment, the women were asked “Which of the following symptoms apply to you at this time?”; and the score per question was chosen as “None” = 0; “Mild” = 1; “Moderate” = 2; “Severe” = 3; “Extremely Severe” = 4 (Table 1).
Statistical analyses
Statistical methods for the original study endpoints have been previously described [25,26]. Analyses were performed for the total pooled population and for subgroups stratified by body mass index (BMI; <25 and ≥ 25) or by ethnicity (European and Chinese). Pooled data were analyzed using an analysis of covariance (ANCOVA) model with treatment, study and treatment-study interaction as factors and the respective baseline values as covariates.
Results
Study population
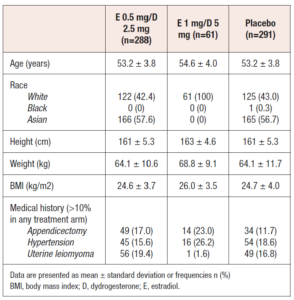
A total of 640 women from the two studies were included in the pooled analysis. Of these, 288 had received E 0.5 mg/D 2.5 mg, 61 had received E1 mg/D 5 mg and 291 had received placebo. Baseline demographics and characteristics for the pooled population are shown in Table 2. Final analysis was performed on 285, 59 and 289 patients, respectively.
Health-related quality of life
Total population
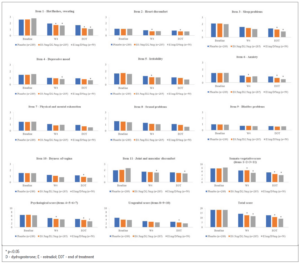
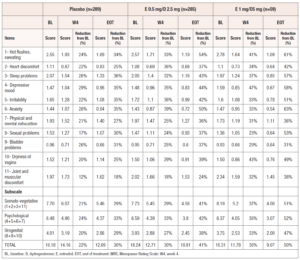
At EOT, the mean MRS scores for items 1 (hot flushes, sweating), 2 (heart discomfort), 3 (sleep problems), 6 (anxiety), and 10 (dryness of vagina) were significantly improved with E 0.5 mg/D 2.5 mg versus placebo, as were MRS total and somato-vegetative, psychological and urogenital combined domain scores (Figure 1). With the exception of items 3 and 10, and the urogenital subscale score, where non-significant improvements could be seen, all of these significant improvements were also seen at week 4 for E 0.5 mg/D 2.5 mg compared to placebo.
For E1 mg/D 5 mg, the mean MRS scores for items 1 (hot flushes), 3 (sleep problems), 4 (depressive mood), 6 (anxiety), and 11 (joint and muscular discomfort), as were the somato-vegetative and psychological subscale scores and the total MRS score were significantly improved versus placebo at EOT (Figure 1). Similarly, with the exception of items 3 and 6 and the psychological subscale scores, all of these significant improvements were seen at week 4 for E 1 mg/D 5 mg compared to placebo.
There was a reduction in all MRS scores (per item, per subscales and total) at week 4 (W4) and EOT versus baseline in both treatment groups (Table 3). All MRS final scores (per item, subscales and total) in the treatment groups at W4 and EOT were numerically lower compared to the placebo group, with the single exception being item 9 (Bladder problems) at W4 for E 0.5 mg/D 2.5 mg group, where the scores were the same. In addition, the reduction from baseline was always higher in the two treatment groups, as compared to the reduction from baseline in the placebo group.
BMI subgroups
In the BMI <25 subgroup, mean MRS scores for items 1, 2, 3, 4 and 10 were significantly improved with E 0.5 mg/D 2.5 mg versus placebo at EOT, as were the somato-vegetative subscale score and the total MRS score [29]. All of these improvements, except the improvements in items 4 and 10, were seen at week 4. With E1 mg/D 5 mg, significant improvements versus placebo were seen for items 1, 4 and 11 and for the somato-vegetative subscale score at EOT. Again, all significant improvements, except the improvement in item 4, were seen at week 4. In the BMI ≥ 25 subgroup, significant improvements versus placebo were seen at EOT for items 1 and 6 with E 0.5 mg/D 2.5 mg, and for items 1 and 6, somato-vegetative subscale score and the total MRS score with E 1 mg/D 5 mg. The improvements at week 4 were not significant in this group.
European and Chinese populations
In the European population, a significant improvement in item 1 was seen with E 0.5 mg/D 2.5 mg vs placebo at EOT [30]. At EOT, with E 1 mg/D 5 mg, significant improvements versus placebo were seen for items 1, 3, 4, 6 and 11 and for the somato-vegetative and psychological subscale scores and also the total MRS. In the Chinese population, significant improvements were seen with E 0.5 mg/D 2.5 mg vs placebo in items 1, 2, 3, 5, 6 and somato-vegetative and psychological subscale scores at week 4 and in all items (except item 8), in all subscale scores (somato-vegetative, psychological and urogenital) and also in the total MRS score at EOT [30].
Discussion
This pooled analysis of two phase 3, randomized, placebo-controlled trials showed consistent improvements in health-related quality of life with low- and ultra-low-dose E plus D versus placebo. The clinically relevant improvements seen in health-related quality of life are likely attributed to the efficacy of low- and ultra-low-dose E plus D in reducing vasomotor symptoms, with significantly lower numbers of hot flushes per day seen with E 0.5 mg/D 2.5 mg versus placebo and E 1 mg/D 5 mg versus placebo in the individual studies [25,26]. In addition, adequate bleeding control seen with E 0.5 mg/D 2.5 mg and E 1 mg/D 5 mg [25,26] is likely to contribute to improved health-related quality of life as perimenopausal bleeding has been shown to have a negative impact on women’s quality of life [31]. Furthermore, the two E plus D formulations have favorable safety profiles with very low rates of treatment discontinuation during the two studies [25,26]. Together, these treatment attributes can positively impact the day-to-day lives of postmenopausal women, reflected in the present analysis as a significant improvement of the total MRS score after 12 weeks of E plus D treatment in this population.
Notably, there were positive health-related quality of life impacts in women with BMI <25 and in those with BMI ≥25, an important finding considering the potential impact of body weight on drug pharmacokinetics and pharmacodynamics [32]. Significant improvements versus placebo in health-related quality of life due to ‘Hot flushes, sweating’ were seen at EOT in both BMI groups. This finding is clinically relevant and highlights the benefit and appropriateness of oral ultra-low dose combination of E plus D, even among women with high BMI. Moreover, a significant improvement versus placebo in health-related quality of life based on the total MRS score was seen at EOT in the BMI <25 subgroup. There were also consistent health-related quality of life improvements across both European and Chinese populations, with significant improvements versus placebo in health-related quality of life due to ‘Hot flushes, sweating’ seen at EOT in both European and Chinese women. Again, the consistency of this improvement across different ethnicities is clinically relevant as the frequency and duration of vasomotor symptoms during perimenopausal transition has been shown to vary based on ethnicity [33-36]. Notably, while numerical improvements were seen in all MRS items in both populations with E 0.5 mg/D 2.5 mg at EOT versus baseline, more of these improvements were statistically significant in the Chinese population compared with the European population. This may be explained by the lower number of patients per group in the European trial compared with the Chinese trial, as well as the differences in ethnicity. Nevertheless, these results from our subgroup analysis demonstrate consistent health-related quality of life benefits, irrespective of BMI or ethnicity. These results can be used to support physicians and patients in clinical decision-making surrounding the use of menopausal hormone therapy.
The strengths of this pooled analysis include the individual patient data from placebo-controlled, double-blind, randomized studies. However, there are some limitations such as the post hoc nature of the analysis and the small number of women in some of the subgroups. Despite these limitations, the analysis provides clinically valuable data regarding the benefits of E 0.5 mg/D 2.5 mg and E 1 mg/D 5 mg in improving health-related quality of life among postmenopausal women, building on the favourable impact shown in previous studies [23-26].
Conclusions
In this pooled analysis of two randomized, placebo-controlled trials, oral, ultra-low dose continuous combined 0.5 mg E plus 2.5 mg D and low dose continuous combined 1 mg E plus 5 mg D improved health-related quality of life across several clinically relevant symptoms assessed with the MRS compared with placebo in postmenopausal women of different range of BMI categories. Additionally, ultra-low dose continuous combined 0.5 mg E plus 2.5 mg D improved health-related quality of life across several relevant symptoms compared with placebo in postmenopausal women of different ethnicities. Given the increasing importance of PROs in relation to clinical benefits, these findings support the use of oral, ultra-low-dose continuous combined 0.5 mg E plus 2.5 mg D and low dose continuous combined 1 mg E plus 5 mg D to improve health-related quality of life among postmenopausal women of different BMI categories and ethnicities.
Data sharing
All relevant data are within the paper and its Supporting Information files.
Statement of authorship
Authors contributed equally to the conception, design and writing of the manuscript. All authors critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Acknowledgments
Editorial assistance was provided by Metamols Ltd, funded by Abbott Established Pharmaceuticals.
Funding
This work was supported by Abbott.
Declarations of interest
EK is an employee of Abbott Laboratories GmbH, Hannover, Germany and owns shares in Abbott. MGC is an employee of Abbott Products Operations AG, Allschwil, Switzerland and owns shares in Abbott. Junyi Yang is an employee of Abbott China, Established Pharmaceuticals Division, Shanghai, China and owns shares in Abbott. MR, QY, and TT declare no conflicts of interest. MGC is an employee of Abbott Products Operations AG, Allschwil, Switzerland and owns shares in Abbott. TS has received consulting fees from Astellas, Gedeon Richter, Mitsubishi Tanabe, Sojournix, Estetra, Actavis, Abbott, Medtronic, Applied Medical, Johnson & Johnson and speaker’s honoraria from Shionogi, Gedeon Richter, Theramex, Abbott, Intuitive Surgical, Applied Medical. REN has on-going relationship with Abbott, Astellas, Bayer HealthCare AG, Besins Healthcare, Exeltis, Fidia, Gedeon Richter, HRA Pharma, Merck & Co, Novo Nordisk, Organon & Co, Shionogi Limited, Theramex, and Viatris. EK is an employee of Abbott Laboratories GmbH, Hannover, Germany and owns shares in Abbott. JCS has received grants/research support from Abbott, Mylan and Pfizer; consulting fees from Abbott, Mitsubishi Tanabe, Mylan and Pfizer; and speaker’s honoraria from Abbott, Bayer, Gedeon Richter, Menarini, Mylan, Pfizer and Viatris.