Introduction
Breast cancer accounts for approximately 15% of all cancer deaths in women, with a lifetime risk of about 1 in 8 [1,2]. More than 75% of breast cancers are either estrogen or progesterone sensitive and would thus respond to endocrine therapy, which includes aromatase inhibitors (letrozole, anastrozole, exemestane), selective estrogen receptor modulators (tamoxifen) and ovarian suppression (GnRH agonists or oophorectomy) [3].
Aromatase inhibitors are used to treat postmenopausal women with hormone receptor-positive early, advanced or metastatic breast cancer after a relapse or disease progression. They inhibit the enzyme aromatase and prevent the conversion of androgens to estrogens, which is the main source of estrogens after menopause. They significantly decrease the incidence of contralateral breast cancer, reduce breast cancer recurrence and improve disease-free survival [4]. They have been shown to be superior to tamoxifen and confer a statistically significant absolute risk reduction of recurrence of 3.6% at 10 years and an increase in overall survival of 2.1% [5]. Aromatase inhibitors are typically used for 5 years but may be extended for up to 10 years in high-risk patients [5].
Osteoporosis is a major cause of morbidity, mortality and health care expenditure [6]. In the general population, women have a 1 in 3 lifetime risk of experiencing an osteoporotic fracture [7]. After menopause, there is an acceleration of age-related bone loss and remodeling resulting in an increased risk of osteoporosis [8].
Female oncologic patients are already at increased risk for osteoporosis and fractures due to the nature of their disease, the presence of comorbidities and the treatment they receive [9,10]. By suppressing estrogen synthesis, aromatase inhibitors enhance bone resorption and further increase the incidence of fractures in this population group. In the MA-17 study and the BIG-98 trial, the aromatase inhibitor letrozole was associated with a higher incidence of osteoporosis (12.2%) than with placebo (6.4%) and a significantly increased risk of fracture [11]. The American Society of Clinical Oncology (ASCO) and Canadian Osteoporosis guidelines recommend that all patients on letrozole are at high risk for osteoporosis and oncologists need to take an expanded role in their regular bone health assessments [12,13].
Adopting healthy lifestyle choices such as exercise, smoking cessation and ensuring adequate calcium and vitamin D intake, reduce fracture risk and are standard of care for osteoporosis prevention [14,15]. Osteoporosis Canada recommends 800 – 2,000 units of vitamin D and 1,200 mg of elemental calcium (through diet and supplements) in postmenopausal patients. Patients who start an aromatase inhibitor also require a baseline DEXA scan, which should be repeated every 1-2 years while on treatment [16-18].
Most guidelines agree that pharmacotherapy for osteoporosis should be initiated for patients with a history of fragility fracture or with a T score of -2.5 or lower. In 2022, the American College of Obstetrics and Gynecology went further to recommend treatment initiation in patients with a T score between -1 and -2.5, who are at increased risk of fracture as determined by a formal clinical risk assessment tool, such as the FRAX calculator [14]. Bisphosphonates such as alendronate, risedronate, zoledronic acid and denosumab are first-line drugs for the treatment of osteoporosis. They reduce fracture risk by 40 – 60%, prevent bone loss and reduce breast cancer recurrence and mortality [19].
Quality Improvement (QI) initiatives & study objective
There is some evidence in the literature that simple Electronic Medical Record (EMR) based interventions can be effective at eliciting change in quality improvement projects [20,21]. In a randomized-controlled trial in 2006, Feldstein et al. evaluated whether EMR-based reminders influence osteoporosis screening and treatment rates and found that the number of patients who received a bone mineral density (BMD) test or osteoporosis treatment was 8-fold higher in the intervention group with reminders [22].
We wanted to calculate the rate of oncologic patients on letrozole in our practice, who receive calcium, vitamin D, and monitoring for BMD and determine if oncologic patients on long-term letrozole are receiving appropriate screening and management for osteoporosis prevention. We also wanted to identify factors associated with adherence to the standard osteoporosis guidelines and to evaluate the impact, if any, of an EMR based reminder at improving the current rate of osteoporosis screening in female oncologic patients receiving long-term letrozole therapy at our institution.
Guidelines and measure of compliance
The standard osteoporosis prevention guidelines used in the typical Canadian Health care setting were reviewed. These included the 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada, the ASCO 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer and the 2022 Clinical Practice guidelines on management of postmenopausal osteoporosis of the American College of Obstetricians and Gynecologists.
For the purpose of this QI initiative, clinicians were deemed to comply with the standard guidelines if there was documented daily vitamin D and/or calcium supplementation in the patient’s chart and there was at least one documented baseline DEXA scan for patients started on long-term letrozole therapy.
Design
This project used the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE) 2.0 guidelines as a framework for design. This is a pre-intervention and post-intervention retrospective chart review of patient medical records at a level III teaching hospital in Ontario, with a capacity of 751 beds. Our QI team consisted of a staff Gynecologic-Oncologist and a senior gynecology resident. Stakeholders from the medical oncology, pharmacy, biostatistics and information technology were engaged throughout the initiative, and helped with the development of the process map, data collection, statistical analysis and implementation of process improvements. The team met regularly to conduct this QI initiative. There were multiple staff meetings, and informational sessions where staff asked questions and their concerns were addressed.
Using the Plan, Do, Study, Act (PDSA) model, the QI team used a fishbone diagram to highlight barriers to suboptimal osteoporosis screening and then created a Key Driver diagram (Figures 1 and 2). Our major intervention was the use of an EMR-based reminder about osteoporosis screening when prescribing letrozole long-term. Documented orders for calcium, vitamin D and BMD testing were the process measure and osteoporosis screening rate was the outcome measure. We implemented one PDSA cycle.
The study consisted of three phases – a baseline preintervention phase, an EMR implementation phase and a post-intervention phase.
Statistical analysis
Continuous variables were described using either median (interquartile range [IQR]) or mean (standard deviations [SD]), based on data distribution, and compared between pre- and post-groups using the Mann-Whitney U test or T test. Categorical variables were reported as counts and percentages, with differences assessed using the chi-square or the Fisher’s exact test. Univariable and multivariable logistic regression models were developed to investigate associations between gender, years of experience, and osteoporosis screening outcomes in pre-intervention data. Additionally, logistic regression models were used to evaluate the effectiveness of the EMR intervention and to identify factors associated with improved guideline adherence and the uptake of calcium or vitamin D supplementation.
Baseline measurements and results
To establish a baseline measurement of current practice around osteoporosis screening, we carried out a retrospective chart review of 446 patients from April 1, 2021, to February 4, 2022. It allowed us to ascertain whether patients were already being screened for osteoporosis as per the standard guidelines. A spreadsheet was kept with deidentified patient details and hospital clinical records were reviewed. A total of 446 charts were examined.
The following inclusion and exclusion criteria were used for our study:
a) Inclusion criteria
- All patients with breast and ovarian cancers who were receiving endocrine therapy with letrozole.
b) Exclusion criteria
- Patients with preexisting osteoporosis prior to starting letrozole.
- Patients with poor prognostic factors and life expectancy < 5 years.
- Patients receiving bisphosphonates for treatment of bone metastases.
- Patients who discontinued letrozole after less than a year of use due to side effects.
- Patients on aromatase inhibitors other than letrozole.
- Deceased patients.
27 patients were excluded from our study because they did not meet our inclusion criteria. Therefore, data was extracted for 419 patients.
The outcome indicators were the following: percentage of patients who received a baseline DEXA scan, percentage of patients who had no DEXA scan documented, percentage of patients who received a baseline DEXA scan and at least one interval surveillance DEXA scan, percentage of patients who were counselled to take calcium and/or vitamin D and percentage of patients who developed osteoporosis on letrozole. The likelihood of adherence to standard guideline recommendations by gender and years of experience were also tracked using univariate and multivariate logistic models.
Patient characteristics are presented in Table 1. The median age of patients in the preintervention group was 67 years, with a median body mass index of 28.3 kg/m2. A 14.6 % of them were current smokers or had smoked cigarettes in the past. While on letrozole, 5.7 % of patients developed osteoporosis.
| Patient characteristics | Pre-intervention group (n=419) |
|---|---|
| Age (median [IQR]) | 67.00 [59.00, 73.50] |
| Cancer type (%) | |
| Breast | 419 (99.8) |
| Ovarian | 1 (0.2) |
| Weight/Kg (median [IQR]) | 73.20 [63.85, 84.60] |
| Height (mean (SD)] | 160.64 (6.88) |
| Body mass index (median [IQR]) | 28.31 [24.70, 32.77] |
| Smokers (%) | |
| Yes | 61 (14.6) |
| No | 358 (85.4) |
| Any BMD testing prior to DEXA (%) | |
| Yes | 17 (4.1) |
| No | 402 (96.9) |
| Received calcium (%) | |
| Yes | 108 (25.8) |
| No | 311 (74.2) |
| Received vitamin D (%) | |
| Yes | 333 (79.5) |
| No | 86 (20.5) |
| Osteoporosis before starting letrozole (%) | |
| Yes | 5 (1.2) |
| No | 414 (98.8) |
| Osteoporosis after starting letrozole (%) | |
| Yes | 24 (5.7) |
| No | 395 (94.3) |
| Recommended family doctor monitors for osteoporosis (%) | |
| Yes | 301 (71.8) |
| No | 118 (28.2) |
| Gender of the MD (%) | |
| Female | 259 (61.8) |
| Male | 160 (38.2) |
| Years of experience of the MD (median [IQR]) | 18.00 [13.00, 20.00] |
Baseline results
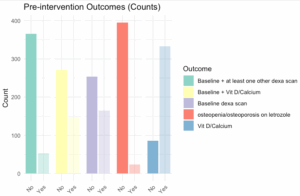
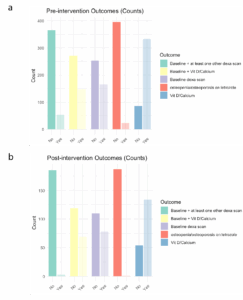
Results of the pre-interventional data analysis are presented in the bar graph (Figure 3). Table 2 summarizes the univariable and bivariable analysis.
| Univariable
OR (95% CI) p-value |
Bivariable
OR (95% CI) p-value |
|
| MD gender: Female | 3.411 (1.616,7.197) <0.01 | 2.91 (1.321,6.409) <0.01 |
| MD years of experience | 0.95 (0.909,0.994) 0.025 | 0.973 (0.928,1.021) 0.265 |
Odds Ratios (OR) – 95% confidence intervals (CI) from Logistic regression models experience. The bivariable model includes only MD gender and MD years of experience.
Baseline interpretation
- Only 39.4% of patients received a baseline BMD density scan.
- Only 35.3% of patients were taking vitamin D and/or calcium and received a baseline BMD testing.
- 5% of patients were counselled to take calcium or vitamin D.
- Only 12.6% of patients received a baseline BMD scan and at least one other interval surveillance BMD scan.
- 7% of patients developed osteopenia/osteoporosis on letrozole.
- Female gender of oncologist provider was associated with a higher odds of adhering to the guidelines in univariable and bivariable analyses.
- Oncologist’s years of experience was not associated with complying with the guidelines.
Statistical analysis of pre-intervention data confirmed that an intervention was necessary to improve the current standard of care surrounding letrozole use and osteoporosis screening. We wanted to find out if a simple EMR based intervention to remind prescribers of guidelines when prescribing letrozole long-term, would be beneficial. To achieve our goals, several strategies were employed. First, brainstorming sessions were held to better understand the barriers and ways to implement the EMR intervention.
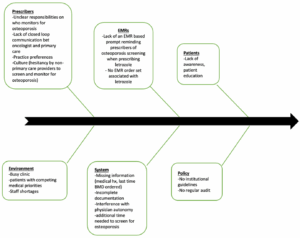
With the help of a fish bone diagram (Figure 1), the QI team analyzed the cause-effect scenario for adherence to osteoporosis guidelines and identified six possible categories of potential root causes contributing to whether a patient receives screening and counselling for osteoporosis when clinically indicated. The categories include prescribers, EMRs, patients, environment, system and policy. Prescriber applies to the clinician’s thought processes and actions. EMRs refers to the versatility and practicality of existing computerized clinical decision support systems such as reminders or an order-set associated with the letrozole prescription. Culture refers to the effect of previous experiences with osteoporosis management in the oncology service. Missing information applies to incomplete or unavailable data when the patient is assessed by the clinician. It was felt that having no EMR reminders, busy clinic, competing medical priorities and unclear responsibilities on who follows up for osteoporosis (i.e. assumption that the patient would undergo monitoring by her family physician) were the main key drivers for failure to screen for osteoporosis.
Key driver intervention
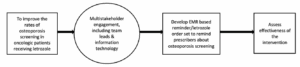
We started our first cycle of QI by implementing an EMR-based modification to remind prescribers about osteoporosis screening when prescribing letrozole. We did not want the stakeholders to feel overwhelmed by multiple system changes at once and chose to implement an EMR based reminder first as it was simple, feasible and measurable. A key driver diagram (Figure 2) shows the logistics involved in screening and prescribing for patients at risk of osteoporosis.
Implementation of the Electronic Medical Record (EMR) based reminder:
The QI team, in collaboration with stakeholders from Information Technology, came up with the idea for an EMR-based reminder and implemented it in a stepwise manner using the PDSA cycle.
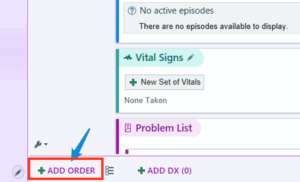
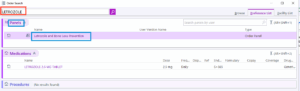
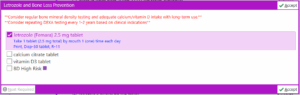
With our EMR modification, by clicking on “+ ADD ORDER” and typing “Letrozole” in the order search (Figure 4 and Figure 5 below), it was possible to either select letrozole by itself as a stand-alone order or as part of a letrozole treatment plan. Selecting the “letrozole and Bone loss prevention” order panel (Figure 5) brings up the letrozole treatment plan whereas clicking on the “letrozole 2.5 mg tablet” opens up the stand-alone order.
If selected, the letrozole order panel will include the letrozole prescription defaulted and calcium/vitamin D and baseline BMD which are not defaulted but selectable. At the top of the panel, in red is also the EMR-based reminder “Consider regular bone mineral density testing and adequate calcium/vitamin D intake with long-term use” and “Consider repeating DEXA testing every 1-2 years based on clinical indications” (Figure 6 and Figure 7).
Post intervention data analysis
Five months after implementation of the EMR-based reminder, another retrospective chart review was performed to assess for the effectiveness of this intervention. The risk of bias in selection of patients was reduced by taking patients directly and sequentially from September 1st 2023, to February 1st 2024, who were initiated on letrozole at our institution for treatment of breast or ovarian cancer.
Out of 195 records in total, 188 patients were included for data analysis. Of the 7 women who were exempt from analysis, 2 were cognitively impaired, 4 discontinued letrozole due to intolerance and 1 had poor functional status. A post-hoc reverse power calculation was performed given the smaller sample size in the post-intervention group compared to the pre-intervention group. This calculated that we were powered to detect a 12% difference.
| Patient characteristics | Post-intervention group (n=188) |
|---|---|
| Age (median [IQR]) | 65.00 [58.00, 72.00] |
| Cancer type (%) | |
| Breast | 186 (98.9) |
| Ovarian | 2 (1.1) |
| Weight/kg (median [IQR]) | 70.35 [62.58, 82.00] |
| Height (mean (SD)] | 160.44 (6.73) |
| Body mass index (median [IQR]) | 27.31 [24.32, 31.53] |
| Smokers (%) | |
| Yes | 40 (21.3) |
| No | 148 (78.7) |
| Any BMD prior to diagnosis (%) | |
| Yes | 6 (3.2) |
| No | 182 (96.8) |
| Received calcium (%) | |
| Yes | 49 (26.1) |
| No | 139 (73.9) |
| Received Vitamin D (%) | |
| Yes | 134 (71.3) |
| No | 54 (28.7) |
| Osteoporosis before starting letrozole (%) | |
| Yes | 0 (0.0) |
| No | 188 (100) |
| Osteoporosis after starting letrozole (%) | |
| Yes | 1 (0.5) |
| No | 187 (99.5) |
| Recommended family doctor monitors for osteoporosis (%) | |
| Yes | 40 (21.3) |
| No | 148 (78.7) |
| Gender of the MD (%) | |
| Female | 132 (70.6) |
| Male | 55 (29.4) |
| Years of experience of MD (median [IQR]) | 13.00 [10.00, 22.75] |
| Pren (%) | Post n (%) | p value | |
|---|---|---|---|
| n | 419 | 188 | |
| Interval between letrozole start and baseline BMD(months) (median [IQR]) | 4.50 [1.00, 13.00] | 2.00 [1.00, 10.75] | 0.016 |
| BMD scan 1 | 61 (14.6) | 3 (1.6) | < 0.001 |
| BMD scan 2 | 18 (4.3) | 0 (0.0) | 0.001 |
| BMD scan 3 | 2 (0.5) | 0 (0.0) | 1.000 |
| No BMD | 251 (59.9) | 110 (58.5) | 0.815 |
| Adherence toGuidelines | 53 (12.6) | 3 (1.6) | < 0.001 |
| Baseline BMD | 165 (39.4) | 78 (41.5) | 0.688 |
| Calcium or VitaminD | 333 (79.5) | 134 (71.3) | 0.035 |
| Baseline DEXA scan and Vitamin D and/or calcium | 148 (35.3) | 69 (36.7) | 0.813 |
n = number of patients in the pre-intervention, post-intervention group and overall; BMD scan 1, 2, 3 – patients who received follow-up BMD date 1, 2, 3; No BMD – patients who had no DEXA scan (BMD) documented; Guidelines – patients who received a baseline DEXA scan and at least one other (interval surveillance) DEXA scan; Calcium or Vitamin D – patients who were counselled to take calcium or vitamin D therapy; Baseline DEXA scan and vitamin D and/or calcium – patients who received a baseline DEXA scan and vitamin D and/or calcium.
We also ran univariable and multivariable logistic regression model for the main and secondary outcomes and the results are shown in tables 5 – 7 below:
| UnivariableOR(95% CI) p-value | MultivariableOR(95% CI) p-value | |
|---|---|---|
| Age of patientsat screening | 1.001 (0.985, 1.017) 0.923 | 1 (0.984, 1.016) 0.978 |
| Patients who are current smokers or have ever smoked | 1.028 (0.666, 1.589) 0.9 | 1.031 (0.661, 1.608) 0.892 |
| MD gender: Female | 2.162 (1.512, 3.09) < 0.0001 | 2.202 (1.515, 3.2) < 0.0001 |
| MD years of experience | 0.99 (0.971, 1.01) 0.323 | 1.004 (0.984, 1.025) 0.694 |
| UnivariableOR(95% CI) p-value | MultivariableOR(95% CI) p-value | |
|---|---|---|
| Age of patientsat screening | 1.006 (0.99, 1.022) 0.464 | 1.005 (0.989, 1.022) 0.527 |
| Patients who are current smokers or have ever smoked | 0.944 (0.603, 1.478) 0.801 | 0.937 (0.594, 1.478) 0.779 |
| MD gender: Female | 2.024 (1.402, 2.92) < 0.001 | 2.016 (1.374, 2.957) < 0.001 |
| MD years of experience | 0.988 (0.969, 1.008) 0.23 | 1 (0.979, 1.021) 0.99 |
| UnivariableOR(95% CI) p-value | MultivariableOR(95% CI) p-value | |
|---|---|---|
| Age of patientsat screening | 1.006 (0.99, 1.022) 0.464 | 1.005 (0.989, 1.022) 0.527 |
| Patients who are current smokers or have ever smoked | 0.944 (0.603, 1.478) 0.801 | 0.937 (0.594, 1.478) 0.779 |
| MD gender: Female | 2.024 (1.402, 2.92) < 0.001 | 2.016 (1.374, 2.957) < 0.001 |
| MD years of experience | 0.988 (0.969, 1.008) 0.23 | 1 (0.979, 1.021) 0.99 |
Bar plots of the measures of outcome, pre-intervention and post-intervention, are shown below for ease of comparison (Figure 8).
Final results and discussion
A total of 607 patients were included in the study over the two phases: 419 patients in the pre-intervention group and 188 patients in the post-intervention group. Patient characteristics by groups are presented in Tables 1 and 3. Demographics were similar between groups.
For the statistical analysis, descriptive statistics were represented as number and percentage, mean and SD or median as appropriate. A p value of < 0.05 was considered statistically significant. A post-hoc power calculation was performed to detect a 12% difference, given the smaller sample size in the post-intervention group. Based on the univariable model results, significant covariates were selected into the multivariable models for main and secondary outcomes.
In the preintervention group, only 39.4% of patients on letrozole had a baseline BMD screening test documented on file. After the EMR-based intervention, there was a very modest increase noted at 41.5%, which was not statistically significant (p=0.688). This shows that our EMR based reminder was not effective as a stand-alone tool to improve the osteoporosis screening rate at our institution.
In our study, 5.7% of patients on letrozole developed osteopenia/osteoporosis in the pre-intervention group (Table 1). The quoted rate of new-onset osteoporosis in patients receiving long-term letrozole is even higher, at about 11% in the literature [22]. This is not surprising, however, as aromatase inhibitors will typically reduce BMD by 1.1 – 3.4%, a rate which exceeds the annual postmenopausal bone loss rate of 1 – 2% [23]. In addition, 21 – 49% of postmenopausal women beginning letrozole therapy might already have baseline osteopenia [23,24]. That is why, the ASCO considers it essential to manage these high-risk patients proactively and recommend annual BMD testing to minimize fracture risks.
Interestingly, when evaluating the study’s primary outcome in a univariate and multivariate analysis, female gender of oncologist was associated with higher odds for adhering with the guidelines (Tables 5 and 7; p<0.001). This agrees with prior published studies which showed that women are more likely to practice guideline-concordant care [25,26].
There was no association between oncologist´s years of experience and guideline compliance (Tables 6 and 7). However, patients seen by more experienced oncologists had reduced odds of taking calcium or vitamin D.
Lessons and limitations
In this QI initiative, we demonstrated that an EMR based reminder, used as a stand-alone tool was not effective enough to improve the osteoporosis screening rate and adherence to standard osteoporosis guidelines recommendations at our institution.
Several important points can be highlighted from this study. First, rates of osteoporosis screening with a BMD test in our cohort continue to remain low at 40%. Multiple barriers likely contribute to implementation failure of the EMR based reminder, including prescribing culture, imperfect electronic medical record interfaces, busy clinics, competing priorities in a sick population, reliance on family physicians for osteoporosis screening and lack of close-loop communications between oncologists and family physicians.
There were various limitations in this study. First of all, both the Canadian Osteoporosis and the ASCO guidelines date from 2010 and 2003 respectively and while still being widely used, it may be prudent to reassess the literature to ensure these guidelines remain current with best practice. Secondly, our study was a retrospective chart review, with lack of randomization and therefore, the possibility of confounding by unmeasured factors. There was also no formal pre-intervention or post-intervention knowledge assessment completed for this project. We only collected verbal feedback from our team. Data collected reflected the five months following the EMR intervention and was collected prematurely to meet the time constraints of a residency program with respect to project submission. Even though a post hoc power calculation was performed because of the disparity in sample size between the pre-intervention and post-intervention data, it may be prudent to review data for instance, in the next twelve months, with a larger sample size, to reassess for any effectiveness of the EMR intervention. Another major limitation was the lack of patient education and involvement regarding the importance of osteoporosis screening in our setting.
We anticipated some resistance from medical providers as it is difficult to deviate from routine practices. In a future PDSA cycle targeting prescriber culture, it may be useful to look at the impact of an osteoporosis guideline drafted and vetted at their local departmental level, incorporated as a link in the Electronic Medical Records, and its impact on provider practice.
In our pre-interventional data analysis, 71.8% of oncologists recommended in their clinic letter that the patient’s family physician monitors for osteoporosis. It would be interesting in another PDSA cycle, to audit the time interval between the initial recommendation and when a BMD testing is actually ordered by the family physician. Delays might be due to failure of timely follow-up by the patient with her family physician, loss of a paper trail, or due to system wait times associated with obtaining a BMD test once ordered. We strongly believe that having a centralized EMR system for patients, where tasks that should be followed up can be flagged to the attention of the respective provider, would go a long way in minimizing delays in follow-up.
Conclusion
Our study demonstrated that an EMR based intervention, as part of a QI initiative, was not effective as a stand-alone tool to improve the rate of osteoporosis screening and adherence to local osteoporosis guidelines by oncologists prescribing letrozole at our local tertiary cancer centre.
Process change necessitates strategic planning and action. Future endeavours will include conducting multi-stakeholder brainstorming sessions looking into the intricacies associated with prescriber culture in a busy clinic with competing medical priorities and to conduct further PDSA cycles as deemed necessary.
Data availability statement
Data is available upon request. The authors, when requested, can provide access to the underlying data supporting the findings present in this manuscript. Kindly contact the corresponding author for inquiries regarding data availability at kevintee@dal.ca
Ethics approval
The Research Ethics Board of Trillium Health Partners, Credit Valley Hospital, determined that this study did not require oversight by its members.
Patient involvement
Patients were not involved in the design, conduct, reporting or dissemination plans of our study.