Introduction
The prevalence of infertility reaches 17.5% worldwide, with primary infertility accounting for 10.5% of cases. Consequently, Early Pregnancy Loss (EPL) stands as one of the most significant concerns in Reproductive Medicine. Its frequency is 13.5%, with the rate escalating to 55% in the case of three consecutive miscarriages. Approximately, 60% of miscarriages occur in the first trimester [1,2]. The causes of spontaneous miscarriages are varied, including genetic and immunological causes, infectious factors, hormonal disturbances, anatomical defects, among others. It must be mentioned, that 30% of couples suffer from unexplained infertility problems [3]. In such cases In Vitro Fertilization (IVF) is often used and its success rate is 30.7% [4]. All patients mentioned above may even conceive, but these pregnancies remain undiagnosed as 60% are lost before the delay of the period and the first determination of blood beta chorionic gonadotropin (β-hCG) [5]. Additionally, it must be noted, that the incidence of EPL increases and IVF effectiveness decreases along with advancing maternal age [6–8].
During pregnancy, complex neuro-endocrinological and immunological mechanisms are activated, contributing to the normal development of pregnancy. Among these processes, one of the key factors is the progesterone-induced blocking factor (PIBF). Specifically, PIBF suppresses myometrial constrictions, reduces the production of pro-inflammatory cytokines, thereby increasing the differentiation and proliferation of T helper cells and blocks the degranulation of natural killer (NK) cells thus reducing their cytolytic function [9].
The scientific attention to PIBF has increased over the last several decades. PIBF consists of 757 amino acids with a molecular mass of 89 kDa [10,11]. There are also shorter forms – 30, 43, and 57 kDa, which are localized in the cytoplasm. These forms are associated with cell-specific intra and extracellular expression [12]. It is thought that the short forms act as PIBF’s receptor ligands [13]. PIBF is produced in the γδ T lymphocytes at the preclinical stage of pregnancy (soon after conception) [14,15]. It must be noted that inhibiting an immune response is a key mechanism for maintaining pregnancy but also may contribute to other pathologies, such as tumour growth, due to local immunosuppression [16]. Szekeres-Bartho et al. first demonstrated that in the lymphocytes of women, who take progesterone (PG), PIBF is produced, which blocks the cytotoxic activity and synthesis of prostaglandin F2α (PGF2α). Thus, in women with threatened preterm delivery, PIBF synthesis is reduced [17]. In other studies, a considerable reduction of PIBF and an increase of pro-inflammatory cytokines – IL-6 and γ interferon (γ-IFN) has been demonstrated in the urine and plasma of women with threatened preterm delivery [18,19]. Pro-inflammatory cytokines are also associated with Recurrent Pregnancy Loss (RPL) and preterm delivery. Besides, PIBF levels are significantly lower in urine and plasma of women with threatened miscarriages [18]. Hereby, Szekeres-Bartho et al. in their study have noted that PIBF helps maintain the normal uterine tone [17]. Therefore, it has become clear that PIBF plays a crucial role in maintaining pregnancy by modulating the immune response. PIBF and PG have immunomodulatory effects on the membrane progesterone receptors (mPR) of CD4+ (Cluster differentiation) T cells. In one study it was concluded that PIBF was able to significantly increase mPR expression on the surface of peripheral CD4+ T cells. As a result, a decrease in PIBF concentration during abnormal pregnancies can modulate mPR expression and the regulatory function of PG on T cells. Consequently, Rafiee M. et al. have concluded that research is necessary to provide a deeper understanding of the etiology of pregnancy loss [20].
PIBF has gained popularity following its detection in various tissues of the reproductive system and more recently, in tumor tissue [12,21,22]. PIBF is also, expressed on the surface of the trophoblast and participates actively in its invasion. Miko et al. have described that PIBF is expressed by the normal placenta, as well as by hydatidiform moles. However, its expression is significantly reduced in complete moles and is not expressed at all in choriocarcinoma [23]. PIBF plays an important role in the maintenance of pregnancy, increasing from the first day of conception [11]. According to Hudic et al. [24], determining the PIBF levels during IVF at the early stage of pregnancy may serve as a predictive marker for the pregnancy outcomes [24].
Therefore, the purpose of our research was to assess the prognostic value of PIBF in early pregnancy loss and examine the correlation between PIBF and PG.
Methods
This prospective observational study included 86 patients and was conducted at “Prof. Zhordania and Prof. Khomasuridze Institute of Reproductology” and “LiderMed” clinic, Tbilisi, Georgia. The study was approved by the local ethical committee and informed consent was obtained from all patients. Biochemical pregnancy (BP) was diagnosed in all patients. 50 patients conceived naturally, and 36 women conceived through IVF. The inclusion criteria were as follows: unexplained infertility, one or more EPL in anamnesis, normal ovulation, and positive β-hCG (>25 mIU/mL) level in the blood on the 12th to 14th day after ovulation and embryo transfer (ET). The exclusion criteria included all causal factors of EPL: tubal, endocrine disorders, ovarian dysfunction, endometriosis, congenital and acquired anomalies of the pelvic organs, confirmed genetical disorders, congenital and acquired thrombophilia, sexually transmitted diseases, acute and chronic inflammatory diseases of pelvic organs, uterine fibroids and polyps, abnormal uterine bleedings, infertility caused by male factors.
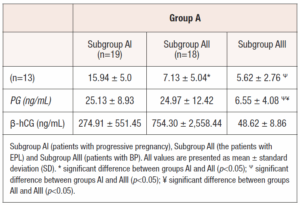
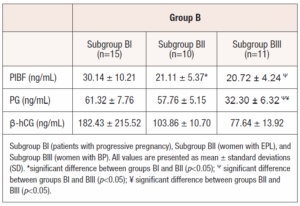
Of the 50 naturally conceived patients (Group A), aged 18-35 (29.50 ± 5.59 years), menstruation began on time in 13 women, which, in our belief, likely indicates that pregnancy was lost at the preclinical stage in those cases. The other 37 women experienced delayed menstruation and clinical pregnancy was confirmed. However, pregnancy loss occurred in 18 women at different weeks of gestation (3-8 weeks). 19 patients had progressive pregnancies, that continued to term delivery. Retrospectively, these patients were divided into three subgroups based on the course of pregnancy: AI (patients with progressive pregnancy, n=19); AII (patients, with EPL, n=18); and AIII (patients with BP, n=13). Similarly, 36 patients who conceived after IVF (Group B), aged 21-35 (30.97 ± 3.78 years), were also divided into three subgroups: BI (patients with progressive pregnancy, n=15); BII (patients with EPL, n=10); and BIII (patients with BP, n=11). In all patients, β-hCG, PIBF and PG were measured in the blood serum on the 12-14th day after ovulation and ET, respectively. The PIBF levels were measured using ELISA kit (Catalogue No.: EH1818). Samples were tested in duplicates, and we ensured strict adherence to the manufacturer's protocols to maintain assay reliability. Statistical analysis was performed using a one-way ANOVA test with SPSS software (Version 26.0 for Windows). Differences were considered significant when p value was <0.05.
Results
Originally, in the naturally conceived women, PIBF levels were significantly higher in patients with clinically confirmed pregnancy (11.65 ± 6.66 ng/mL) compared to those with BP (5.62 ± 2.76 ng/mL, p<0.05). PG levels were significantly higher in patients with clinically confirmed pregnancy (25.05 ± 10.62 ng/mL) compared to those with BP (6.55 ± 4.08 ng/mL, p<0.05). Among IVF patients, PG levels were significantly higher in those with clinically confirmed pregnancy compared to BP (61.10 ± 7.26 vs. 32.30 ± 6.32 ng/mL, p<0.05), whereas no significant difference was found in PIBF levels between these two groups (26.52 ± 9.59 vs. 20.72 ± 4.24 ng/mL, respectively, p>0.05).
After follow-up and dividing the clinical pregnancy groups into subgroups (progressive pregnancy and EPL), the results were as follows: In the naturally conceived women (Subgroup AI), the mean level of PIBF was significantly higher in patients with progressive pregnancy (15.94 ± 5.0 ng/mL) compared to patients with EPL (7.13 ± 5.04 ng/mL) and BP (5.62 ± 2.76 ng/mL, p<0.05), but no significant difference was found in PIBF level between women with EPL and BP (p>0.05, Table 1). (Table 1)
Similarly, after IVF, PIBF was statistically higher in patients with progressive pregnancy (30.14±10.21 ng/mL) than in the EPL (21.11 ± 5.37 ng/mL, p<0.05) and BP subgroups (20.72 ± 4.24 ng/mL, p<0.05), but no significant difference was found in PIBF levels between women with EPL and BP (p>0.05, Table 2). (Table 2)
In the naturally conceived women, mean PG levels were significantly higher in the patients with progressive pregnancy (25.13 ± 8.93 ng/mL) as compared to women with BP (6.55 ± 4.08 ng/mL, p<0.05). PG levels were also significantly lower in the patients with BP as compared to those with EPL (24.97 ± 12.42 ng/mL, p<0.05).
In IVF women, PG levels were not statistically different between progressive pregnancy (61.32 ± 7.76 ng/mL) and EPL (57.76 ± 5.15 ng/mL) subgroups, (p>0.05). However, PG levels were significantly lower in the BP subgroup (32.30 ± 6.32 ng/mL, p<0.05).
Additionally, the mean levels of PIBF and PG level were statistically significantly higher in the IVF subgroups than in the appropriate naturally conceived women subgroups (AI vs. BI; AII vs. BII; and AIII vs. BIII, p<0.05).
There was no statistically significant difference in β-hCG levels between groups and subgroups. There was no significant correlation between PIBF and PG levels in the subgroups AI (r = -0.04, pP>0.05), AIII (r = -0.16, p>0.05), BI (r = 0.42, p>0.05), BII (r = -0.12, p>0.05), BIII (r = 0.30, p>0.05), except subgroup AII, where a moderate negative correlation was found (r = -0.64, p<0.05).
Discussion
The prevalence of infertility worldwide is relatively high, estimated at 17.5%, indicating that approximately one in every six adults experience infertility [25]. Several studies suggest that primary infertility rates are higher in various countries compared to secondary infertility rates, with figures ranging from 6% to 16% (average 10.5%) for primary infertility and approximately 2% for secondary infertility [26,27]. However, from 1990 to 2010 the rate of secondary infertility was higher than primary: 8.7-32.6% vs. 0.6-3.4%, respectively [28]. The rates mentioned above, are confusing and it is essential to consider, that in developing countries, they may be much higher. All data pertain to clinically approved pregnancies. However, considering the potential number of pregnancies lost before the delay in menstruation, the rate could significantly increase, which is already alarming. The cause of infertility, at least in half of cases, is often undetectable and referred to as “unexplained infertility” [29]. Theoretically, those patients may still conceive, but these pregnancies are lost within the first two weeks of pregnancy, remaining undiagnosed. In our view, the majority of these cases are linked to the immune responses of women, which can become activated after conception. One such factor is PIBF.
PIBF levels begin to rise from the early stages of conception and continue to increase throughout pregnancy [11]. As noted by Macklon et al., the greatest losses occur at the preimplantation or early implantation stages [5], which underscores the critical role of PIBF in supporting pregnancy progression. The significance of PIBF is particularly notable in IVF. Monitoring PIBF levels during IVF can serve as the predictive indicator of pregnancy outcomes [24].
In a study assessing the effects of dydrogesterone on hormonal profiles and PIBF concentrations in women facing the threat of miscarriage, the findings indicated that dydrogesterone-induced elevation of PIBF could potentially enhance pregnancy outcome [30]. Additionally, low levels of PIBF have been identified as predictive of preterm delivery occurring between 24 to 28 weeks of gestation [31]. Given the lack of diagnostic markers for preimplantation and early implantation stages, the rate of undiagnosed pregnancy and consequently, the rate of EPL remains very high. For this reason, we aimed to assess the prognostic value of PIBF in EPL, in both naturally conceived women and those who conceived via IVF.
Our clinical trial is innovative due to its unique study design, which sets it apart from previous studies in this field. Specifically, the pre-assessment of PIBF and PG levels, followed by an analysis based on longitudinal observation and pregnancy development, introduces a novel approach. While our primary results reflect an initial exploration, they are significant and were derived from a robust statistical analysis, regardless of the sample size.
The findings from our study align with those of Lim et al., indicating that PIBF levels are notably higher in naturally conceived women with ongoing, progressive pregnancies compared to women experiencing EPL or BPs. This correlation supports the notion that PIBF levels increase throughout the trimesters in healthy pregnant women [11]. Additionally, Szekares-Bartho et al., have reported that PIBF synthesis is reduced in women with threatened preterm delivery [17].
Quite interesting was our study result concerning PIBF, which was similarly lower in both EPL and BP subgroups compared to the progressive pregnancy subgroup, with no statistically significant difference between those two subgroups. This finding suggests that a low PIBF level in the preclinical stage of pregnancy could potentially serve as an indicator of miscarriage risk, not only in cases of BP but also in clinically recognized pregnancies. Our results are proven in other studies. According to Polgár et al. PIBF was one of the most important factors associated with pregnancy outcome, as its concentrations in both urine and plasma increase with pregnancy progression. Conversely, in cases of miscarriage or preterm delivery, they observed a lack of the expected rise in PIBF levels [32]. Sahin et al., have found significantly lower PIBF levels in women with unexplained infertility compared to the fertile control group [33]. These findings align with our results, as our study included patients with unexplained infertility and with a history EPL.
In contrast to PIBF, our study found that PG levels were significantly lower in the BP subgroup compared to the EPL subgroup, and there was no significant difference in PG levels between progressive pregnancy and the EPL subgroups. This suggests that PG levels may not be as informative in predicting EPL, especially in instances where EPL occurred at 5-8 weeks of gestation, despite relatively high PG levels in naturally conceived patients.
Our results differ from those of Ku et al., who reported that serum PG levels increase linearly during 5-13 weeks of gestation and that low PG levels are associated with a threatened miscarriage and a complete miscarriage at 16 weeks of gestation [34].
According to one study, PIBF is released by the lymphocytes in the presence of PG, and the percentage of these lymphocytes increases in the luteal phase [35]. Both PG and PIBF are promising biomarkers for predicting pregnancy viability [11]. However, we found no linear correlation between PIBF and PG levels during the preclinical stage of pregnancy, suggesting that other factors may contribute to PIBF regulation. This finding highlights the complexity of the underlying mechanisms and the need for further research.
Our findings are consistent with those of Check et al., who found that the corpus luteum is not a reliable sign for producing PIBF [36]. The same tendency was maintained in our study, after the IVF procedure - the PIBF level was higher in women with progressive pregnancy than in patients with EPL and a BP. Thus, high PIBF levels may point to a favorable outcome after IVF. Our suggestion is confirmed in a study by Adamczak et al., who found that higher PIBF-1 concentrations in follicular fluid may indicate a greater possibility of successful IVF [37]. Hudic et al., consider that PIBF measured early in pregnancy predicts outcome in women undergoing IVF procedures [24].
In the IVF subgroup of our study, similarly to naturally conceived women, PG levels were significantly lower in BP compared to the EPL subgroup, with no significant difference observed between progressive pregnancy and the EPL subgroups. Additionally, following the IVF procedure, PG was significantly higher in the women with clinically confirmed pregnancies than in those with BP, while PIBF levels showed no significant differences between clinical and BP.
Ku et al. have found that low PG and PIBF concentrations in blood predict spontaneous miscarriage among women with threatened miscarriages between 6-10 weeks of gestation [38]. Our study produced similar results concerning PIBF levels, which were significantly lower before delayed menstruation in patients with BP compared to those with clinical pregnancy in the group of women who naturally conceived. However, after follow-up and dividing the group into subgroups, the difference between BP and EPL subgroups was not observed. Whereas, in IVF patients, PIBF levels were not statistically different in women with a clinical and BP as originally so after dividing into subgroups.
Interestingly was the finding that PIBF and PG were statistically higher in IVF subgroups than in the naturally conceived women subgroups, which may be related to the hormonal therapy used in IVF cycles. Despite this, the prognostic trend of PIBF in naturally conceived women and those undergoing IVF was similar, as it was similar for PG levels.
Based on our results, we may conclude that PIBF levels are more sensitive than PG in EPL. While PG levels remained high even in early miscarriages, PIBF levels showed a significant difference, suggesting its potential role as a prognostic marker for early pregnancy loss. However, repeated measurements could provide additional insights, as logistical constraints limited us to a single time point in this study. Future research will aim to include longitudinal assessments to better understand PIBF dynamics.
Additionally, despite the normative ranges provided by kit manufacturers, they are still limited and primarily for research purposes. The normal reference ranges for PIBF in clinical practice have not yet been clearly established. Our study underscores the critical need for further investigation to define these ranges and integrate them into routine clinical practice.
While these markers are undoubtedly important, it is crucial to acknowledge the potential influence of other factors contributing to unexplained infertility and EPL. We believe that identifying trends and setting directions for future research is of paramount importance, particularly in areas where existing studies are limited.
In conclusion, PIBF emerges as a potential and interesting prognostic indicator for EPL, encompassing even its preclinical stage. PG may be considered a prognostic marker for clinical pregnancy.
Funding
None.