Introduction
Polycystic ovary syndrome (PCOS) is a major cause of menstrual irregularities, anovulation and androgen excess in women [1]. This condition represents one of the most common endocrinopathies in women, affecting between 5 and 10 percent of women, depending on the population studied and the applied diagnostic criteria [1]. The clinical manifestations of PCOS are heterogeneous and tend to change throughout the patient's life. Among the more frequently reported features we have reproductive disturbances (e.g. menstrual irregularities, anovulation, pregnancy complications), signs of hyperandrogenism (e.g. acne, hirsutism, alopecia), metabolic dysfunctions (e.g. dyslipidemia, insulin resistance, MetS), and psychological symptoms (e.g. anxiety, depression, low self-esteem) [2].
The different clinical features of PCOS led to the proposal of multiple diagnostic criteria over the years. According to the latest recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome, the diagnostic criteria from 2018 International Evidence-based Guideline criteria, which was built on the consensus based 2003 Rotterdam criteria, are recommended for precise PCOS diagnosis [3-5].
PCOS and the diagnostic criteria
To establish a diagnosis of PCOS at least two of the following three criteria are needed: 1) irregular menstrual cycles or absence of ovulation, 2) clinical signs of biochemical evidence of hyperandrogenism, 3) polycystic ovarian morphology [4].
Menstrual irregularities in PCOS typically begin during the peripubertal period, being menarche sometimes delayed. The menstrual pattern commonly includes oligomenorrhea (fewer than nine menstrual periods per year) and, less frequently, amenorrhea (absence of menstrual periods for more than three consecutive months) [6]. Women with PCOS often experience more regular menstrual cycles after the age of 40, following the physiological reduction in ovarian reserve [6].
Most women with PCOS show both clinical and biochemical evidence of hyperandrogenism.
Signs of hyperandrogenism often manifest as hirsutism, acne, male-pattern hair loss, or seborrhea. Hirsutism is typically assessed using the Ferriman-Gallwey score, with the diagnostic threshold varying depending on the patient's ethnicity [6,7]. When isolated, acne may not necessarily indicate hyperandrogenism, as it is often common during adolescence. However, the presence of moderate inflammatory acne with more than 10 facial lesions, or severe acne during the perimenarcheal years, suggest a correlation with hyperandrogenism {8]. Hair loss is an unusual manifestation of hyperandrogenism in adolescents and it is usually evaluated with the Ludwig visual scale [9]. When it occurs, it may follow both a male-pattern of distribution (affecting fronto-temporo-occipital areas of the scalp) or a female-pattern of distribution (starting from the crown of the head) [10]. Biochemical hyperandrogenism is usually evaluated dosing total and free testosterone plasma levels [5]. If testosterone levels are not elevated other hormones such as androstenedione and dehydroepiandrosterone sulphate should be assessed [5].
Polycystic ovarian morphology (PCOM) represents the typical polycystic appearance of the ovaries when observed with transvaginal or transabdominal ultrasound [5]. In patients with PCOS, ovaries are characterized by an abundance of preantral and early antral follicles (ranging from 2 to 9 mm in size), usually with a peripherical localization [11]. The criteria for ultrasound evaluation of the ovaries have evolved over the years, reflecting the increased diagnostic accuracy of modern imaging equipment. The latest recommendations from 2023 define PCOM if at least one of the following two characteristics is present: 1) follicle number per ovary ≥ 20 in at least 1 ovary, 2) ovarian volume ≥ 10 mL or follicle number per section ≥ 10 in at least 1 ovary [5]. These criteria are currently only validated for adult subjects (at least eight years after menarche); there are, however, no universally accepted criteria for evaluating PCOM in adolescents [5].
The 2023 recommendations also introduced anti-Müllerian hormone (AMH) as an alternative to ultrasound to define PCOM in adults [5]. AMH, which is produced by antral and preantral ovarian follicles, is usually higher in patients with PCOS because of simultaneous activation of multiple antral follicles [12]. Despite being a useful tool for PCOM assessment, there is currently no internationally validated standard value for this parameter [5]. However, some meta-analyses suggest using a threshold of AMH > 4.7 ng/mL to identify PCOM [13].
The diagnosis of PCOS is confirmed once other conditions with similar characteristics have been excluded such as non-classical congenital adrenal hyperplasia (NCCAH), Cushing's syndrome, thyroid disorders, androgen-producing tumors, or hyperprolactinemia [5].
As the diagnoses of PCOS is established with the presence of either two or all three criteria from the Rotterdam Consensus, in 2012 four specific PCOS phenotypes have been identified [14].
Phenotype-A is often referred to as the “complete” PCOS phenotype as it fulfils all the diagnostic criteria for PCOS while phenotype-B does not require the presence of PCOM [15]. Both phenotypes-A and B are often defined as “classic” PCOS and these women tend to have an increased risk of insulin resistance, dyslipidemia, hepatic steatosis and the onset of the MetS if compared with other phenotypes [15,16].
Phenotype-C is also called the “ovulatory” PCOS as only clinical/biochemical hyperandrogenism and PCOM are present [15]. Finally, phenotype-D, also known as “non-hyperandrogenic” PCOS, consists in only menstrual irregularities paired with PCOM. These patients usually have milder clinical manifestations [15].
PCOS, insulin resistance and the MetS
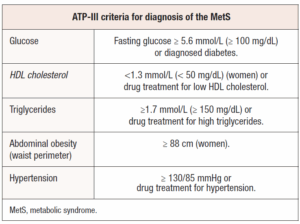
Between 40 and 85% of women with PCOS are overweight or obese compared to age-matched controls. Moreover, both lean and obese patients have a higher prevalence of insulin resistance; although this parameter is not included among the diagnostic criteria for the syndrome [17]. Clinical features of insulin resistance include acanthosis nigricans, obstructive sleep apnea, hepatic steatosis or MetS onset [2]. The MetS is diagnosed when various risk factors are present such as abdominal obesity, dyslipidemia, glucose intolerance, and hypertension, which together indicate a higher likelihood of cardiovascular diseases and increased risk of mortality [18]. The prevalence of the MetS in PCOS ranges from 33.4% to 47% while it affects up to 24% of the general population in Europe [19-22].
Various international organizations and expert groups have suggested different diagnostic criteria for the MetS [23,24]. The most commonly used guidelines are those from the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) which define the MetS as the presence of at least three of the traits reported in Table 1 [25]. (Table 1)
Given the hyperandrogenic state and metabolic alterations in women with PCOS, it is essential to carefully consider these factors when tailoring therapeutic options for optimal treatment. Treatment strategies should address all aspects of the syndrome while also considering the patient’s potential desire for future childbearing. These options can be divided into two main categories: non-pharmacological interventions (recommended to all patients) such as lifestyle modifications and psychologic support and pharmacological interventions, which can be further categorized based on the patient's desire to pursue pregnancy or not [5,6].
Among the pharmacological interventions proposed for PCOS patients we have hormonal contraceptives, insulin sensitizers (metformin or supplements such as carnitines, inositols or α-lipoic acid), antiandrogens (flutamide, finasteride, spironolactone and CPA) or drugs that induce ovulation (clomiphene citrate, letrozole or gonadotrophins injections) [5,26].
If the patient with PCOS does not have a short-term desire for pregnancy, hormonal contraceptives represents the first-line therapy for managing both menstrual abnormalities and signs of hyperandrogenism [5].
The present narrative review aims to provide valuable insights to guide in the selection of the most suitable hormonal contraception options for patients with PCOS, who are often characterized by increased metabolic risk factors.
Combined hormonal contraceptives
Combined hormonal contraceptives (CHCs) are birth control methods that contain both estrogen and progestin. They regulate ovarian function by acting on the hypothalamic-pituitary axis, reducing gonadotrophins secretion, and suppressing ovarian stimulation. While both hormones contribute to these effects, progestins play a key role in contraception through additional mechanisms. These include thickening of cervical mucus to make it less permeable to sperm, impairing normal tubal motility, and inducing decidualization or atrophy in the endometrium, making it less suitable to embryo implantation [27]. On the other hand, the estrogen component of CHCs, when taken orally, increases serum concentrations of thyroxine-binding globulin (TBG), cortisol-binding globulin (CBG), and sex hormone-binding globulin (SHBG). In particular, the elevation of SHBG reduces the levels of free serum testosterone, helping to alleviate clinical signs of hyperandrogenism [28]. In addition, estrogens reduce receptor expression in specific areas such as the endometrium, improving the stabilizing effect of the progestogenic component [28].
CHCs represent the first-line treatment for the most common PCOS clinical manifestations and are available in various forms such as COC pills, transdermal patches and vaginal rings [28,29].
The latest international evidence-based guideline for PCOS recommends the use of COCs in reproductive-aged adults with PCOS to manage hirsutism and irregular menstrual cycles [5]. However, these guidelines do not provide specific recommendations regarding the dosage, or the preferred particular estro-progestin formulations to be used in these patients but it does affirm that there is no clinical benefit in using high-dose ethinylestradiol (≥ 30 μg) compared to low-dose ethinylestradiol (< 30 μg) for treating hirsutism in adults with PCOS [5]. Additionally, it is suggested to consider natural estrogen preparations or the lowest effective ethinylestradiol doses for adolescents with PCOS, limiting the use of the 35 μg ethinylestradiol plus CPA formulation as a second-line therapy compared to other COCs [5].
When choosing a contraceptive for patients with PCOS, various factors should be taken into account, including body weight, menstrual patterns, clinical signs of hyperandrogenism, and the presence of hyperinsulinemia or the MetS. The most suitable CHC should be selected by carefully considering both the estrogen and progestin components based on their specific properties and how they align with the patient's individual needs [29].
Progestin component
Progestagens, or progestogens, include both progesterone, the natural hormone produced by the ovaries, and synthetic steroids known as progestins, which are designed to replicate the actions of endogenous progesterone [30]. Progestin drugs can be classified into four different generations based on the time they were introduced to the market [31]. The first generation of progestins includes norethisterone acetate, the second generation consists in norgestrel and levonorgestrel (LNG), the third generation comprises gestodene, desogestrel, and norgestimate, while in 4th generation we have DRSP and DNG [32].
When categorized by structural properties, progestins can be grouped into three major categories. The first group includes molecules derived from C-21 progesterone, such as NOMAC, CPA, and chlormadinone acetate (CMA). The second group consists of progestins derived from C-19 nortestosterone, DNG, LNG, norgestimate, desogestrel, etonorgestrel, and gestodene. The final group includes derivatives of spironolactone, which consists solely of DRSP [32].
The structural differences among progestogens result in varying interactions with the body’s steroid receptors. While all progestogens typically bind to the progesterone receptor (PR), the newer generations also tend to interact with other receptors, including the androgen receptor (AR), estrogen receptor (ER), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR) [30].
The affinity of progestins for various receptors can vary significantly. Older progestins like LNG retain androgenic activity, while others such as gestodene, norgestimate, and desogestrel exhibit minimal or no androgenic effects. In contrast, newer progestins such as DNG, CPA, DRSP, and NOMAC have anti-androgenic properties [31,33].
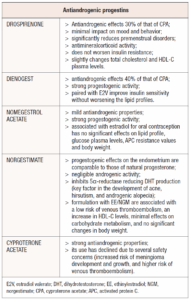
Since the clinical manifestations of PCOS are often associated with hyperandrogenism, the ideal progestin for contraception in these patients should be one with minimal or no androgenic activity. Among the options, CPA offers the strongest antiandrogenic effect while DNG and DRSP have antiandrogenic effects that are approximately 40% and 30% of that of CPA, respectively [34]. These progestins have varying effects on metabolic profiles, and these differences should be carefully considered, especially since women with PCOS tend to have a higher incidence of metabolic impairments [34]. Moreover, all of them exist on the market associated with ethinylestradiol, but only few are offered in combination with natural estrogens, such as estradiol (E2), estradiol valerate (E2V), or estetrol (E4) [29].
Let’s discuss about the progestins that, due to their specific characteristics, appear to be the most suitable for treating patients with PCOS (Table 2).
Drospirenone (DRSP)
DRSP is an analogue of 17-α-spironolactone and therefore represents an antimineralcorticoid progestin [34}]. It is quickly absorbed when taken orally, reaching peak plasma levels within 1-2 hours, and has a half-life of 27 hours. It has minimal binding to SHBG and therefore has a high bioavailability of 76% [36]. In addition to binding to the PR, DRSP also interacts with the MR and the AR, making it one of the most effective progestins available for PCOS management [35,37].
This 4th generation progestin is highly effective in counteracting hyperandrogenism, helping to reduce symptoms such as acne and seborrhea. Its anti-androgenic effects are well-documented in vivo, with a potency approximately one-third of that of CPA, when administered to castrated, testosterone-substituted male rats {38,39].
DRSP also has minimal impact on mood and behavior compared to other progestins. Moreover, it has been shown to significantly reduce the incidence of premenstrual disorders and related mood disturbances, making it a preferred option for patients who are concerned about these side effects [40,41].
One of DRSP’s main benefits is its antimineralcorticoid activity, which counteracts the effect that oral estrogens have on the synthesis of angiotensinogen in the liver. This activity helps preventing the increase in aldosterone that leads to water and salt retention. As a result, DRSP can reduce bloating and minor weight gain often associated with COC intake and may contribute to a slight reduction in blood pressure [36].
Although DRSP may cause slight changes in total cholesterol and HDL-C plasma levels, it does not exacerbate insulin resistance in women with PCOS, thereby alleviating some metabolic alterations [42].
Dienogest
DNG is a 19-nortestosterone derivative that is not alkylated at C17 [31]. It is rapidly absorbed after oral administration, reaching plasma peak levels within 2 hours, with a half-life of 9.1 hours [43]. Despite being a nortestosterone derivative, DNG does not exhibit androgenic activity; instead, it has antiandrogenic effects, with approximately 40% of the potency of CPA, the most potent antiandrogenic progestin [34]. In vivo studies performed on male rats also suggest that DNG’s antiandrogenic properties may surpass those of other progestins such as DRSP [39].
DNG has negligible binding to SHBG, which results in a higher concentration of free available plasma progestin and a bioavailability of approximately 95% [43]. Additionally, DNG displays strong progestogenic activity, making it highly effective in treating conditions like endometriosis or abnormal uterine bleeding by inducing significant endometrial atrophy [44].
When paired with E2V, DNG has shown to improve insulin sensitivity without worsening lipid profile, thus, making it a viable contraceptive option for PCOS patients with metabolic impairments [45].
Nomegestrol Acetate (NOMAC)
NOMAC is a derivative of 19-norporgesterone and is characterized by its rapid absorption, with serum concentrations peaking approximately 4 hours after oral administration. Additionally, it has a long half-life, ranging from 35 to 50 hours [46]. NOMAC primarily circulates bound to albumin and does not bind to SHBG, ensuring a high concentration of the free molecule in the bloodstream [31].
This progestin lacks both androgenic and mineralocorticoid effects but can competitively block androgen receptors, exhibiting mild antiandrogenic properties [46,47]. In addition, NOMAC has strong progestogenic activity due to the absence of a CH3 group at the 19 position, which enhances its affinity to the PR and enables effective endometrial control [48]. NOMAC has been associated with the inhibition of endometrial vascularization and the downregulation of both PR and ERs, thereby limiting estrogen-induced endometrial proliferation [46]. NOMAC is a potent antigonadotropic agent, capable of suppressing ovulation at a daily oral dose of at least 1.25 mg [47,49]. Moreover, when this progestin is associated with E2, for oral contraception, no significant changes in lipid profile, glucose plasma levels, APC resistance values and body weight has been observed [50].
Norgestimate (NGM)
NGM is a 3rd generation progestin derived from 19-nortestosterone. It acts as a prodrug, with liver microsomes rapidly converting it into LNG (10%) and norelgestromin (90%) [51,52]. After oral intake, NGM reaches peak plasma concentrations within 30 minutes, has a terminal half-life between 45 and 71 hours and does not bind to SHBG [53].
The progestogenic effects of NGM and its metabolites on the endometrium are comparable to those of natural progesterone. Moreover, both NGM and its main active metabolite, norelgestromin, exhibit minimal interaction with ARs, resulting in negligible androgenic activity [51,54].
Recent research has shown that NGM can inhibit 5α-reductase, the enzyme that converts testosterone into dihydrotestosterone a key factor in the development of acne, hirsutism, and androgenic alopecia [54]. NGM is marketed in combination with ethinylestradiol for contraceptive use in two formulations: a monophasic preparation (providing 35 μg of ethinylestradiol and 250 μg of NGM daily for 21 days) and a triphasic formulation (providing varying doses of NGM across three phases) [54].
Contraceptives containing ethinylestradiol/NGM are associated with a low risk of venous thromboembolism, an increase in HDL-C levels without significantly impacting the lipid profile, minimal effects on carbohydrate metabolism, and no significant changes in body weight [54].
Cyproterone acetate (CPA)
CPA is a synthetic progestin known for its exceptionally strong antiandrogenic properties. It derives from 17α-hydroxyprogesterone and can used in combination with EE in combined oral contraceptives. Due to its potent ability to inhibit androgens activity, CPA can also be used in hormone therapies for transgender women and in the treatment of hormone-sensitive cancers such as prostatic cancer [55].
However, its use has recently declined, particularly in Italy, due to several safety concerns such as increased risk of meningioma development and growth as well as a higher likelihood of venous thromboembolism onset [56,57].
Estrogenic component
The estrogenic component in CHCs significantly participates to enhance the contraceptive efficacy by inhibiting gonadotropin release, thus, preventing ovulation. Additionally, it stabilizes the endometrial lining, ensuring more regular menstrual cycles, and reducing irregular bleeding [33]. This estrogenic support also addresses the decrease in estrogen levels that results from ovulation suppression [58].
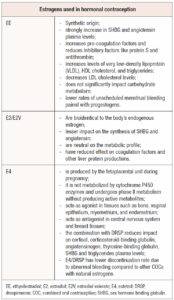
Ethinylestradiol is the primary estrogen used in CHCs, and it is widely paired with most progestins available on the market [59]. However, due to the associated dose-dependent risks of thromboembolism and cardiovascular issues, over the years there has been a push to replace ethinylestradiol with natural estrogens like E2, E2V and E4 to enhance the safety and tolerability of these contraceptives (Table 3) [33].
Ethinylestradiol is structurally distinct from E2 because of an ethinyl group attached to carbon 17, which remains during its metabolism, preventing its conversion to E2. In contrast, E2V, with a valerate group at carbon 17, is easily converted back into E2 after oral absorption. As for E4, it has four hydroxyl groups compared to the two in E2 and does not convert back to E2 during metabolism representing a terminal metabolism product [59].
Ethinylestradiol
When taken orally, approximately 90% of ethinylestradiol is absorbed in the upper gastrointestinal tract and undergoes first-pass metabolism [60]. After absorption, ethinylestradiol is further metabolized into 3- and 17-sulfates, which are partially deconjugated back into ethinylestradiol during enterohepatic recirculation [61]. A 30-µg oral dose of ethinylestradiol typically has a half-life of 8.4 ±4.8 hours, while a 1.2 mg topical dose has a significantly longer half-life of 27.7 ± 34.2 hours [60,61].
Due to its high estrogenicity and metabolism in the liver, ethinylestradiol significantly influences hepatic protein production, increasing the synthesis of SHBG, angiotensinogen, cortisol-binding protein, coagulation factors, and lipoproteins [33]. The rise in SHBG levels induced by ethinylestradiol, coupled with the ovarian suppression caused by contraceptive use and the potential anti-androgenic effects of various progestins, can help manage clinical symptoms of hyperandrogenism [5]. However, ethinylestradiol also alters the coagulation system, promoting pro-coagulation factors and reducing inhibitory factors like protein S and antithrombin, which raise the risk of venous thromboembolism [62].
The use of CHCs containing ethinylestradiol does not appear to be linked to significant weight gain but can lead to changes in lipid profiles. Ethinylestradiol, specifically, tends to increase levels of VLDL-C, HDL-C, and triglycerides, while decreasing LDL-C. Because of this, monitoring lipid profiles is important during contraceptive use, particularly in individuals that have a high cardiovascular risk [63]. Nevertheless, studies have shown that oral contraceptives containing ethinylestradiol do not significantly impact carbohydrate metabolism in patients with PCOS [64].
Aside from its synergistic antiandrogenic effects, the high estrogenicity and potency of ethinylestradiol are useful as they result in lower rates of unscheduled menstrual bleeding if compared to formulations containing natural estrogens like E2/NOMAC [65].
Estradiol (E2) and Estradiol valerate (E2V)
E2V differs from E2 due to the presence of a valerate group at the carbon 17 position, and is readily converted to E2 after absorption. Both E2 and E2V are terminally metabolized into estrone (E1) and estrone sulphate (E1S) [34,66]. In contraceptive formulations, E2 is available on the market in combination with NOMAC, while E2V is commonly paired with DNG [34].
Natural estrogens like E2 and E2V offer several benefits over ethinylestradiol in CHCs. Being bioidentical to the body's endogenous estrogen, they have a more targeted effect on ERs, which helps minimize unwanted interactions and side effects. They also have a lesser impact on the synthesis of SHBG and angiotensin, reducing fluid retention and blood pressure fluctuations. Additionally, they present a lower risk of cardiovascular complications being neutral on the metabolic profile and have reduced liver toxicity, as they are less likely to affect coagulation factors and other liver functions [33].
Estetrol (E4)
E4 is a natural estrogen synthesized by the human fetal liver during pregnancy. Its metabolism in human liver cells is distinct from that of other estrogens as it is not operated by Cytochrome P450 enzymes and it undergoes phase II metabolism without producing active metabolites [37]. E4 is significantly less potent than ethinylestradiol, with 18–20 times lower activity, but it is orally bioavailable and has a long half-life exceeding 24 hours [34].
Unlike other estrogens, E4 selectively stimulates nuclear estrogen receptor alpha (ER-α) without activating the membrane ER-α. This enables E4 to function as an agonist in tissues such as bone, vaginal epithelium, myometrium, and endometrium, while acting in the presence of E2 as an antagonist in other ones such as the central nervous system and breast tissues [58].
E4 is marketed in combination with DRSP as an oral contraceptive, offered in a 24-day active pill regimen followed by 4 placebo days. This combination has demonstrated several advantages over ethinylestradiol-based CHCs, including reduced impacts on cortisol, corticosteroid-binding globulin, angiotensinogen, TBG, SHBG and triglycerides. Additionally, compared to other COCs containing natural estrogens, E4/DRSP has shown a lower rate of discontinuation due to abnormal bleeding [67].
Combined oral contraceptives (COCs) and PCOS
COCs, in addition to lifestyle modifications, are the first line treatment for women with PCOS who do not wish to conceive, as they can regulate menstrual cycles and alleviate symptoms of hyperandrogenism [5]. The progestin component suppresses luteinizing hormone (LH) secretion, reducing ovarian androgen production, while the estrogen component increases SHBG plasma levels, thus lowering serum free androgen concentrations [29].
Before initiating COC therapy in PCOS patients, it is crucial to thoroughly assess their medical history, focusing on cardiometabolic risk factors like hypertension, dyslipidemia, smoking, glucose intolerance, and diabetes, as well as conditions like migraine and depression. A detailed family history, especially regarding thromboembolic events and diabetes, should also be obtained [68]. Additionally, baseline measurements, including body mass index (BMI), waist circumference, blood pressure, and hirsutism score, are necessary and should be paired with blood exams such as an oral glucose tolerance test and lipid profile to assess baseline cardiometabolic risk [29].
Hormonal contraceptive prescriptions should follow the eligibility criteria established by the World Health Organization (WHO), which were last updated in 2015 [69]. Before usage, patients should be informed about potential side effects, including gastrointestinal issues, breast tenderness, headaches, and mood changes, as well as the rare but serious risks of venous thromboembolism or arterial thrombosis [29].
The latest guidelines for PCOS management do not favor one COC formulation over another, but they suggest considering natural estrogen preparations and the lowest effective estrogen doses (e.g. 20-30 µg of ethinylestradiol or its equivalent) to balance efficacy and minimize metabolic risks [5].
When managing hyperandrogenism, progestins with stronger anti-androgenic properties, such as DRSP, DNG, NOMAC or NGM are preferable [29]. The COC combining 35 µg of ethinylestradiol with CPA should be reserved as a second-line therapy, in cases of moderate to severe hirsutism or acne, due to its higher risk of adverse effects [5]. For addressing hirsutism, a treatment duration of at least six months is recommended to evaluate the response [70].
Ethinylestradiol-containing COCs may be preferred over hormonal contraceptives with natural estrogens to increase SHBG levels and further reduce free plasma androgens. However, they may also negatively affect glucose tolerance [71]. For this reason, progestins with minimal metabolic side effects, like DRSP or DNG, and natural estrogens should be the first choice in overweight/obese women or those with concerns about glucose tolerance [71].
Most COC regimens follow a 28-day cycle with 21 days of active hormone pills and a 7-day placebo or pill-free interval. However, recent research suggests that a 24+4 regimen, with a shorter four-day hormone-free interval, may be more beneficial for PCOS patients with hyperandrogenism. A longer seven-day break has been associated with increases in androgenic hormones like androstenedione, testosterone, and 17-hydroxyprogesterone, potentially exacerbating symptoms of hyperandrogenism. Therefore, a shorter hormone-free interval may offer better control of these symptoms in PCOS patients [72].
Vaginal ring and transdermal patch and PCOS
COCs remain the most popular method of birth control, but they have certain limitations. First of all, daily oral intake is required, which can lead to issues with adherence, second, these contraceptives also undergo hepatic first-pass metabolism, which may lead to increased side effects [73]. To address these challenges, non-oral contraceptive methods, such as transdermal patches and vaginal rings, have been developed [74].
Transdermal contraceptive patches are designed to provide stable plasma hormone levels over time. This method bypasses the liver's first-pass metabolism, reducing the likelihood of systemic side effects and allowing for the use of lower hormone doses. By avoiding gastrointestinal absorption and subsequent hepatic metabolism, transdermal patches minimize the risk of drug interactions and enzyme degradation that can occur with COCs. This makes transdermal patches particularly advantageous for women with metabolic disorders, as they tend to have a lower impact on lipid profile and other metabolic parameters [75,76].
Currently, only a limited number of transdermal contraceptive patches are available [77]. In Italy, the ethinylestradiol/norelgestromin patch, is the primary available option, delivering 20 μg of ethinylestradiol and 0.15 mg of norelgestromin daily [78]. Norelgestromin is the active metabolite of NGM, and it primarily targets the PR while exhibiting minimal androgenic activity [78]. This makes the ethinylestradiol/norelgestromin patch a suitable choice for women with conditions characterized by androgen excess, such as PCOS, especially for those who struggle with daily drug intake [78]. This makes the ethinylestradiol/NGM patch a suitable choice for women with conditions characterized by androgen excess, such as PCOS, especially for those who struggle with daily drug intake [78].
The patch is applied once a week, offering a more convenient option compared to daily pills. The steady hormone release provided by the patch helps reduce estrogen-related side effects like nausea and menstrual migraines, which are often associated with high peak levels of estrogens [77]. However, transdermal patches can sometimes cause localized skin irritation, and a higher incidence of breast discomfort compared to oral contraceptives [79].
In addition to transdermal patches, the vaginal ring is another non-oral contraceptive device that offers distinct advantages. In the European market, one of the available formulations of the vaginal ring includes a combination of 15 µg of ethinylestradiol and 120 µg of etonogestrel, which is the active metabolite of desogestrel [74]. This device releases hormones directly through the vaginal mucosa, minimizing the impact of first-pass liver metabolism and leading to lower systemic exposure to ethinylestradiol than oral contraceptives [80]. This direct absorption route reduces the potential for systemic side effects and is associated with fewer metabolic disturbances, making it a viable option for women with the MetS [80].
Unlike oral contraceptives, the vaginal ring has not been associated with significant changes in total cholesterol or HDL-C levels, though there may be a slight elevation in triglycerides [80]. Moreover, the monthly dosing schedule of the vaginal ring is easier for many women to adhere when compared to the daily regimen and can therefore improve compliance [80].
Recently Mosorin et al. [81] explored the metabolic effects of oral contraceptives compared to vaginal contraceptives in women with PCOS and found that both methods were effective in reducing androgen levels, with minimal impact on glucose metabolism and lipid profiles [81]. This suggests that non-oral contraceptives can be a good option for women with PCOS, offering effective contraception with potentially fewer metabolic side effects.
Progestin only contraceptives in PCOS women
Progestin-only contraceptives are commonly prescribed for women who cannot tolerate or have contraindications to COCs [82]. These methods include progestin-only pills (POPs) and long-acting reversible contraceptives (LARCs), such as the LNG-releasing intrauterine device (IUD) and the subcutaneous etonogestrel implant [83].
Over time, these contraceptives can lead to endometrial atrophy and amenorrhea, though they may also cause spotting or breakthrough bleeding [84]. POPs typically contain a daily dose of 75 µg desogestrel, except for DRSP-only pill, which includes 24 active days of 4 mg DRSP followed by 4 placebo days [84].
Although limited research exists on the effects of POPs and LARCs in women with PCOS, these methods do not appear to negatively affect cardiometabolic health and seem to offer sufficient endometrial protection [5,85].
However, progestin-only contraception generally has minimal impact on hyperandrogenism in PCOS patients, except for the DRSP-only pill due to its anti-androgenic effects [35]. For this reason, in cases where androgen excess persists, or when the patient cannot take estrogens due to obesity or other risk factors for CHCs, combining progestin-only contraception with anti-androgen medicaments may be an effective approach [5,29].
Antiandrogens and insulin sensitizers added to hormonal contraception
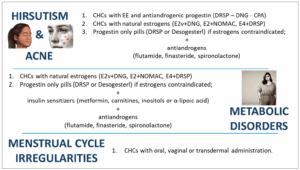
Managing patients with PCOS requires a personalized approach, especially when selecting contraceptives that balance clinical needs with comorbidities and metabolic alterations (Figure 1). Contraceptives may not be sufficient to address all clinical signs, particularly hyperandrogenism, and additional treatments with antiandrogenic drugs such as spironolactone, CPA, flutamide or finasteride can be beneficial in combination with effective contraception to avoid teratogenic risks [5].
Flutamide, for example, acts as an AR antagonist and can help improve skin conditions like acne and hirsutism by blocking residual androgen effects [86]. Finasteride acts by inhibiting 5α-reductase, preventing the conversion of androgens to dihydrotestosterone, thus, primarily impacting hirsutism [87,88]. However, both flutamide and finasteride carry the risk of hepatotoxicity, though this can be minimized using low doses [89].
Spironolactone, commonly used in adolescent PCOS patients, is an aldosterone antagonist with antiandrogen effects. It is typically administered at a dosage ranging from 25 to 100 mg daily and is usually well tolerated, with mild, dose-dependent side effects like metrorrhagia, polyuria and abdominal discomfort [5,86,90].
Although CPA is effective in reducing hyperandrogenism, it is generally avoided due to its association with increased risks, including meningioma development [5].
The addition of antiandrogens to contraceptive therapy is usually considered after at least six months if results are unsatisfactory, as improvements in skin conditions like acne and hirsutism may take time due to the skin’s natural renewal cycle of around 100-120 days [91]. For metabolic management, insulin sensitizers such as metformin, alpha-lipoic acid, and inositols are commonly used in conjunction with contraceptives or alone [5,26].
Metformin, widely used for type 2 diabetes, can be beneficial for PCOS patients with a BMI ≥ 25 kg/m², improving metabolic and anthropometric parameters [5]. A combination of metformin and COCs may provide additional benefits in high-risk metabolic patients, addressing both hyperandrogenism and menstrual irregularities [5,86]. When using metformin, starting at a low dose (e.g. 500 mg daily) and gradually increasing to 2,000-2,500 mg per day is recommended to enhance tolerance [5].
Additional integrative therapies such as alpha-lipoic acid or D-chiro-inositol, are alternatives for patients who cannot tolerate metformin, offering insulin resistance reduction with fewer side effects [26].
Conclusions
PCOS requires a personalized treatment approach, as its manifestations vary widely among patients. While not all women with PCOS are overweight, the syndrome's impact on body weight, metabolic health, and androgen excess must guide our therapeutic choices. Treatment plans should consider each patient's unique characteristics, their metabolic status, manifestations of hyperandrogenism, and personal goals, such as menstrual regulation, weight control or aesthetic improvement desire.
A wide range of contraceptive options is available for managing PCOS symptoms. COCs with progestins like CPA, DNG, NOMAC, DRSP, or NGM paired with estrogens (ethinylestradiol, E2, or E4) offer effective control of hyperandrogenic symptoms and endometrial protection. Non-oral options such as the vaginal ring, transdermal patches, or progestin-only contraceptives, while offering the regulation of menses, they are generally less effective for treating acne, hirsutism, and seborrhea.
When androgen excess persists, the addition of anti-androgen medications, such as spironolactone, flutamide or finasteride, may be necessary to achieve optimal results, especially when natural estrogens or progestin-only options are selected due to comorbidities.
Metabolic considerations should always be factored into treatment. Insulin sensitizers, such as metformin, combined with lifestyle modifications, are crucial for managing insulin resistance and reducing the long-term risks associated with PCOS and hormonal treatments. By tailoring treatments to each patient's unique needs, clinicians can more effectively manage the varied aspects of PCOS and help patients improve their quality of life.
Funding
None.