Introduction
Liguria is a north-western region of Italy with about 1.5 million people living in four provinces; the province of Savona has a population of about 280,000. This district has several secondary and primary public health service endocrinology centres, but no primary centre. Data on the prevalence of positive thyroid antibodies postpartum [1] and preliminary data on the management of hypothyroidism in pregnancy in Liguria [2] were published in 2008. Recently, data on iodine load [3] and thyroid volume [4] in Savona and the nearby district of Genoa have also been published. While the therapeutic management of hyperthyroidism in pregnancy in Liguria has been investigated in a multicentre study [5], to our knowledge no data are currently available on the real-world management of thyroid hypofunction in pregnancy.
It is well known that pregnancy has a considerable impact on thyroid hormone metabolism [6], thyroid gland size [7] and iodine requirements [8]. The high reserve capacity of a normal thyroid gland allows the biological stress of pregnancy to be overcome. Subclinical hypothyroidism (SCH) —defined as an elevated thyrotropin (TSH) concentration with normal serum levels of free-thyroxine (f-T4) —affects at least 10% of women [9, 10]. In pregnancy, the prevalence of SCH depends on the TSH reference range used, and may reach 25% [11-14]. Thyroid dysfunction in pregnant women is almost always caused by chronic autoimmune thyroiditis [12, 15]. Overt hypothyroidism (OH; TSH concentration >10 mU/l, with f-T4 below the reference value) is less frequently (<1%) diagnosed in pregnancy [16-18]. OH and SCH in pregnancy have been addressed in several guidelines and statements [19-25]. These have focused on the early detection and management of thyroid hypofunction in order to prevent potential adverse maternal and foetal outcomes. In 2004, it was suggested that previous levothyroxine (L-T4) dosages should be increased by about 30% [20]. Subsequently, trimester-specific TSH ranges were reported [21] and it was suggested that L-T4 should maintain TSH levels <2.5 mIU/l in the first and second trimesters and <3.0 mU/l in the third trimester [22]. More recently, a wider range of normal TSH levels was proposed, if no institutional normal range is available, and greater attention to the presence of thyroid autoimmunity was recommended [25]. In a recent meta-analysis, an increase in miscarriage was associated with inadequate control of hypothyroidism (TSH levels >4 mIU/l); however, higher rates of gestational diabetes mellitus (GDM), pre-eclampsia and premature delivery seemed to be caused by L-T4 over-treatment [26]. Moreover, the adherence of physicians to statements and guidelines may be suboptimal [20-25]; consequently, the actual management of SCH/OH in pregnancy may differ from the ideal management. We analysed the long-term management of thyroid hypofunction in a single secondary centre located in the Savona district, in order to evaluate adherence to specific recommendations. The study covered pre-gestational examinations, L-T4 treatment to maintain adequate TSH levels, adherence to scheduled endocrine examinations in pregnancy, and maternal and foetal outcomes.
Materials and methods
Subjects.
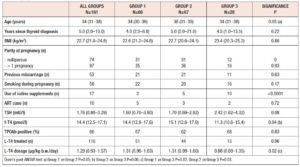
Subjects were identified from medical records at the Endocrine Unit of Priamar Clinical Diagnostic Centre referring to the period 1999 to 2019. Exclusion criteria were: age less than 18 years; twin or higher-order gestations; administration of other medications known to affect thyroid function during pregnancy; and lack of an e-mail address. We selected 129 women examined for Hashimoto’s thyroiditis (HT) and other thyroid dysfunctions during pregnancy. Pre-conception thyroid diagnoses were: HT (n=68), SCH with negative thyroid antibodies (n=26), uni-nodular or multi-nodular goitre (n=25), post-surgical hypothyroidism (n=9), and congenital hypothyroidism (n=1). Two thyroid cancer patients were also enrolled because they had been disease-free for a long time (undetectable thyroglobulin with negative thyroglobulin antibodies; negative neck ultrasonography). In all women, demographic variables, parity, smoking habits, iodine intake and current therapies were recorded (Table 1); 161 pregnancies were monitored. After confirmation of pregnancy, gestational age was determined from the date of the last menstrual period, adjusted according to ultrasound (US) findings, and expressed in full weeks. The pregnancies were subdivided into three groups according to when they were monitored: before 2011 (group 1, n=66), between 2011 and 2017 (group 2, n=67), and from 2018 to the end of data collection (group 3, n=28). Twenty-five women were evaluated for 2 (n=20), 3 (n=4), or 4 (n=1) pregnancies. One woman had 2 pregnancies in two different groups. Pre-conception examination was not performed in 36 out of 161 pregnancies. In these pregnancies pre-conception data were collected retrospectively. In 12 pregnancies, the outcome was not available. Twenty-one women were not on L-T4 at the time of pre-conception examination. All subjects on/starting L-T4 were instructed to take the drug on an empty stomach and not to take other drugs concomitantly. Adherence to this regimen was confirmed at each subsequent contact/visit. As this evaluation was retrospective, no formal request was made to the Liguria Ethics Committee. All women gave written consent for data collection.
Protocol.
At the pre-pregnancy visit, women were informed of the need to start L-T4 or increase their L-T4 dosage when pregnancy was confirmed. According to the year in which pregnancy began, hypothyroidism was managed in accordance with the available literature data. In Group 1 pregnancies, women were advised that the amount of L-T4 would be increased by 20-30% [20]; in groups 2 and 3, women were advised that the amount of L-T4 would be increased step by step to maintain TSH at the level recommended by current guidelines [22, 25]. After the first adjustment, L-T4 was increased in these two groups also according to the increase in body weight (b.w.), with an arbitrary increase of 25 µg/week per 2 kg b.w. increase. An iodine intake of >150 µg/day was recommended. The first clinical and hormonal examination was performed at week 8 (median; interquartile range, IQR weeks 3–11) in 157 pregnancies. In 4 pregnancies, early miscarriage occurred (weeks 4–8). After week 8, women periodically (generally every 4–6 weeks) underwent only gynaecological examinations. During pregnancy, endocrine surveillance was conducted by e-mail. Women were asked to send in their clinical data (estimated week of gestation, b.w., arterial pressure, L-T4 dosage, changes in other therapies), thyroid data (TSH, f-T4, total-T4 if available) and clinical biochemistry data (full blood count, glycaemia and urinalysis). A median of approximately 4 (IQR: 3-5) serum samples were obtained from each woman. When excluding women with early miscarriage (n=4), women with subsequent spontaneous/therapeutic interruption of pregnancy (n=15), and women lost to follow-up (n=6), the mean number of thyroid tests increased from 3.6 (±1.6 SD) to 4.0 (±1.4).
At the end of the gestational period, all women were invited to reduce their L-T4 dosage and to undergo clinical examination 1–2 months after delivery. At this examination, data on the delivery and the neonate at birth were also collected. New data on thyroid function (f-T4, TSH) and thyroperoxidase antibody (TPOAb) levels were examined, to judge the need for changes in L-T4 therapy. Any further information regarding the health of the baby was evaluated.
Outcomes.
In this study, the primary aim was to evaluate pregnant women’s adherence to a regimen that maintained TSH in a gestational normal range according to current guidelines. We also evaluated overall compliance with the endocrinologist’s advice. Thyroid function was monitored post-partum to evaluate the consequences of L-T4 treatment. The secondary aim was to evaluate delivery data. Pre-term delivery was defined as birth before the 37th week of gestation. Miscarriage was defined according to Kolte et al. [27]. Biochemical pregnancy loss was defined on the basis of reduced/negative βhCG; early miscarriage was defined as the loss of an embryo before the 10th week. Recurrent pregnancy loss (RPL) was defined as a history of 2 clinical miscarriages, not necessarily consecutive. Stillbirth was defined as birth of an infant that had died in the uterus after the 20th week of gestation. Neonatal admission was defined as the admission of a neonate to the neonatal intensive care unit (NICU), mainly due to foetal distress or icterus.
Laboratory examinations.
Serum samples were analysed in the Public Health Service laboratory of the Azienda Sanitaria Ligure 2 (ASL 2) in Savona (Italy) by means of electrochemiluminescence immunoassay, using the Cobas automated analytics platform (Roche Diagnostics, Mannheim, Germany). Non-pregnancy normal ranges are 12.0 to 22.0 pmol/l for f-T4 and 0.3 to 4.2 mIU/l for TSH. The functional sensitivity of the TSH assay is 0.03 mIU/L, with intra- and inter-assay coefficients of variation of 3% and 7%, respectively. No trimester-specific TSH ranges were available. From 2017 onwards, normal TSH reference values were taken from the available literature [25, 28]. Several commercial methods were used for TPOAb evaluation during the study period, and negative TPOAb values were assigned according to the normal ranges reported by the manufacturers. Total T4 (TT4) was evaluated by radioimmunoassay only in private laboratories, which centralized the results to a reference centre in Northern Italy. Non-pregnant normal TT4 reference levels are 45–110 µg/l. According to the American Thyroid Association (ATA) 2017 guidelines [25], a clinically acceptable TT4 range in pregnancy can be calculated by raising the non-pregnant limit by 50% after the 16th week of pregnancy. Before that time (weeks 7–16), the normal TT4 range was calculated according to Weeke et al. [29]. When available, urinary iodine concentration (UIC) was used as a biochemical marker of iodine status. UIC was quantified in morning spot urine samples by means of commercial colorimetric methods, as already reported [3]. In our regional area, the non-pregnant median UIC level is 101.0 µg/l (IQR 67.0-145.8 µg/l).
Statistical Methods.
Body mass index (BMI) was calculated on the basis of the weight (kg) and height (m) reported in the medical files, according to the formula: kg/m2. A high gestational weight gain was defined as a total gain >24.5 kg. Smoking was stratified as yes/no (yes meant a minimum of 5 cigarettes daily; women who stopped smoking during pregnancy were also regarded as smokers). We based the diagnosis of HT on the documentation of TPOAb positivity and a heterogeneous hypoechoic pattern on US examination. SCH was defined as a serum TSH level from 4 mIU/l to 10 mIU/L with normal serum f-T4. OH was defined as a TSH level >4 mIU/l with decreased f-T4 concentration, or TSH >10 mIU/L, regardless of f-T4 level. Definitions were taken from the 2017 ATA recommendations [25]. Gestational hypertension was defined as new-onset hypertension without proteinuria with blood pressure >140/90 mmHg after 20 weeks’ gestation [30]. GDM was defined according to the standard diagnostic criteria established by the American Diabetes Association [31]. Categorical variables were described as percentages, and continuous variables as mean, SD, median and IQR. Continuous variables were compared by means of the two-tailed Kruskal-Wallis analysis of variance, Mann-Whitney test, and Wilcoxon test when applicable. For categorical variables, percentages were compared by means of Chi-square tests. P values <0.05 were considered statistically significant. All statistical analyses were performed by means of GraphPad 8.4.0 software (San Diego, CA, USA). Data collection and subsequent analysis were performed in compliance with the Helsinki Declaration.
Results
Clinical data and thyroid data.
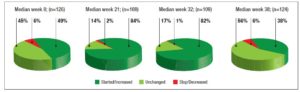
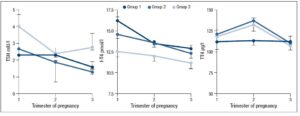
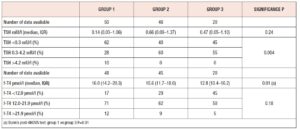
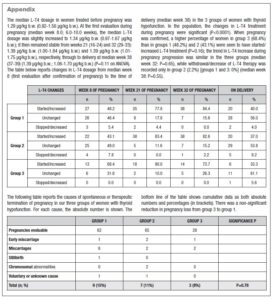
No differences in time from thyroid diagnosis, BMI, parity, previous miscarriages, smoking habits and pre-pregnancy assisted reproductive technology were noted among the 3 pregnancy groups (Table 1). Instead, slight but significant differences in age were noted (P=0.05). From <2011 to 2019, a small number of women were instructed to take iodine supplements before pregnancy, but this number increased over time (P<0.001). The data showed a decreasing trend in exogenous L-T4 treatment over time when thyroid dysfunction necessitated this therapy before the beginning of pregnancy (Table 1). Figure 1 shows the percentages of subjects in whom L-T4 was started/increased, unchanged or stopped/decreased during pregnancy (see also Appendix). In the whole population, changes in L-T4 treatment were significant (P<0.0001) during pregnancy. At the first evaluation after confirmation of pregnancy (median week 8), a higher percentage of women in group 3 than in groups 1 and 2 were seen to have started/increased L-T4 treatment (P=0.16); the trend in L-T4 increase during pregnancy progression was similar in the three groups (median week 32: P=0.65), while withdrawal/decrease of L-T4 therapy was recorded only in group 2 (2.2%) (median week 38: P=0.55; see also additional material). In the 1st trimester, TSH values were available in 86% of evaluable pregnancies (median 1.87 mIU/l; 0.82–3.80 mIU/l), f-T4 in 80% (15.2 pmol/l; 12.9–17.5 pmol/l) and TT4 in 31% (112.5 µg/l; 92.3–130.0 µg/l). In the 2nd and 3rd trimesters, TSH values were available in 82% (median 1.75 mIU/l; 0.92–2.83 mIU/l) and 79% (median 1.20 mIU/l; 0.52–2.22 mIU/l) of pregnancies, f-T4 in 77% (13.3 pmol/l; 11.2–15.2 pmol/l) and 71% (12.1 pmol/l; 10.0–14.5 pmol/l) and TT4 in 33% (124.0 µg/l; 112.0–147.0 µg/l) and 24% (127.0 µg/l; 105.3–141.3 µg/l). Comparison of trimesters showed a significant downward trend in TSH (P<0.002) and f-T4 (P<0.0001) and a significant increase in TT4 (P=0.01) levels. On post-ANOVA, TSH levels were lower in the 3rd trimester than in the 1st (P=0.02) and 2nd (P=0.02), f-T4 levels were higher in the 1st trimester than in the 2nd (P<0.001) and 3rd (P<0.001), and TT4 levels were lower in the 1st trimester than in the 2nd (P=0.01). TPOAb levels were recorded during pregnancy in 88% of pregnancies in which a history of delivery (n=137) was available. In 4 pregnancies, TPOAb levels changed from positive to negative. Figure 2 reports changes in TSH levels during pregnancy in the 3 groups (ANOVA, P=0.03). TSH levels were significantly lower in group 1 than in group 3 at the 1st evaluation (P=0.03) and remained significantly lower in groups 1 (P=0.03) and 2 (P=0.02) than in group 3 at the evaluation during the 3rd trimester. At the first evaluation during pregnancy, TSH levels <0.3 mIU/l were noted more frequently in groups 1 (16%) and 2 (9%) than in group 3 (0%), while TSH >4.0 mIU/l was recorded more often in group 3 (38%) than in groups 1 (19%) and 2 (19%). During the 3rd trimester, TSH levels <0.3 mIU/l were noted only in groups 1 (17%) and 2 (19%), but not in group 3 (0%), while TSH levels >4.0 mIU/l decreased from group 1 (12%) to groups 2 (4%) and 3 (4%). Free-T4 levels differed widely among the groups during the 1st (P<0.0001) and 3rd trimesters (P=0.05) (Figure 2). These levels were lower in group 3 than in the other groups at the 1st evaluation (vs group 1: P<0.0001; vs group 2: P=0.004), and remained significantly lower than in group 1 (P=0.04) at the last evaluation. In all groups, the percentages of women with hypothyroxinaemia increased significantly from the 1st trimester (group 1: 6%, group 2: 17%, group 3: 48%; P<0.0001) to the 3rd trimester (group 1: 32%, group 2: 55%, group 3: 68%; P=0.02). In groups 1 (P=0.001) and 2 (P<0.0001), but not in group 3 (P=0.17), the percentage of women with hypothyroxinaemia was higher in the 3rd trimester than in the 1st trimester. Slight hyperthyroxinaemia was noted only at the 1st evaluation during pregnancy in groups 1 (4%) and 2 (10%). TT4 was not routinely evaluated; the frequency of this evaluation decreased from group 1 (45% of all pregnancies) to group 3 (25%). No difference in TT4 values was noted among the groups at the first and last examinations during pregnancy, but during the 2nd trimester TT4 was significantly lower in group 1 than in group 2 (P=0.006) (Figure 2).
Obstetric and neonatal outcomes.
The outcome of pregnancy was unknown in 4 and 2 cases in groups 1 and 2, respectively. In groups 1, 2 and 3, pregnancy was interrupted in 15%, 11% and 8% of cases, respectively (P=0.78). The causes of pregnancy termination are detailed in the additional material. Only in a single group 1 woman were two consecutive pregnancy losses recorded. GDM was diagnosed during pregnancy in 11%, 9% and 16% of women in groups 1, 2 and 3, respectively (P=0.98). Gestational hypertension and pre-eclampsia were observed in 4%, 2% and 4% of women in groups 1, 2 and 3, respectively (P=0.77). Maternal b.w. on delivery was documented in 48% of full-term pregnancies. Total median weight gain was 14 kg (IQR 11–17 kg) in group 1, 14 kg (9–16 kg) in group 2 and 12 kg (9–14 kg) in group 3 (P=0.20). A high gestational weight gain was observed in just one case, in group 2. The mode of delivery was reported in 130 medical records: vaginal (n=89; 67%) and spontaneous (n=81; 62%) in the majority of cases; caesarean section in 33% (n=43). In groups 1, 2 and 3, delivery was vaginal in 76%, 62% and 67% of pregnancies (P=0.61) and by cesarean section in 24%, 38% and 33%, respectively (P=0.26). In 136 pregnancies, the median week of delivery was week 38.0 (IQR 37.0–39.0): 37.0 (37.0–39.0), 38.0 (36.5–39.0) and 39.0 (37.5–39.0) in groups 1, 2 and 3, respectively (P=0.18). Premature birth (gestational age <37 weeks) was reported in 25%, 25% and 21% of cases in groups 1, 2 and 3, respectively (P=0.89). Only 2 pre-term newborns in group 1 were admitted to the NICU with respiratory distress. Neonatal birth weight was slightly lower in group 2 (median 3.15 kg; IQR 2.79–3.37 kg) than in groups 1 (3.27 kg; 2.91–3.66 kg) and 3 (3.24 kg; 3.09–3.60 kg) (P=0.06). The APGAR score was reported in 71%, 78% and 88% of medical files (P=0.26). The median (9.0) values of 1-minute scores were similar in the three groups, with an IQR of 9–10 in groups 1 and 2, and 9–9 in group 3. The 5-minute values of median (10.0) scores and IQR (10–10) were the same in the three groups.
Post-partum maternal and newborn outcome.
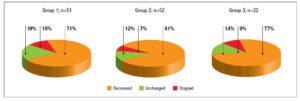
Post-partum information was available in the majority (n=125) of pregnancies with known outcome (n=130): in 100%, 95% and 92% in groups 1, 2 and 3, respectively. The median time from delivery to examination was 10 weeks (IQR 7–16) in 109 records, without significant differences among groups [group 1: 11 weeks (7–16), group 2: 11 weeks (7–18), group 3: 7 (6–11); P=0.14]. There was a significant (P<0.001) increase in BMI (n=93) from pre-pregnancy (22.6 kg/m2; 20.5–24.5 kg/m2) to post-partum examination (23.9 kg/m2; 21.2–26.8 kg/m2). In 88% of women, the median L-T4 dosage was 1.27 µg/kg b.w. (IQR 0.92–1.62 µg/kg b.w.). Figure 3 shows the percentages of women in whom L-T4 was decreased, unchanged or stopped after delivery. No differences in percentages were noted among the groups (P=0.80). Hormonal data on post-partum examination are reported in table 2. No significant difference in TSH levels was noted among the groups, while a significant (P=0.01) decrease in f-T4 levels from group 1 to group 3 was observed. Dunn’s post-ANOVA test documented significantly lower f-T4 levels in group 3 than in group 1 (P=0.01). The percentage of women with TSH in the normal range and without subclinical or clinical hyperthyroidism increased from group 1 to group 3. Overt hypothyroidism and isolated hypothyroxinaemia were noted only in group 1 and group 3, respectively (Table 2).
Clinical information on offspring was reported in the records of 120 mothers, with a lag >1 year from delivery in 103 cases (median lag 4 years; IQR 2–7 years). Offspring malformations and post-natal diseases were reported in 8 males. In pregnancies in group 1, post-natal neuropsychiatric diseases were reported (autism spectrum disorder, epilepsy, attention-deficit/hyperactivity disorder). In two newborns in group 2, reflux nephropathy requiring nephrectomy, and a congenital cholestatic liver disease of unknown aetiology and requiring liver transplantation were reported; one patent ductus arteriosus is still under evaluation. In group 3 newborns, a single umbilical artery and a congenital blepharoptosis were reported.
Discussion
While optimal control and treatment of OH in pregnant women are mandatory, in order to reduce the associated potential risks, the use of an interventional approach in SCH is still debated [32]. Elevated TSH levels seem to be associated with pregnancy loss [15, 33], prematurity [33] and neonatal distress syndrome [34]. However, large randomized clinical trials have failed to show any improvement in the cognitive function of offspring born to L-T4-treated SCH mothers [35]. A review by Maraka et al. [36] confirmed that SCH in pregnancy was associated with multiple maternal and neonatal adverse outcomes, but failed to ascertain a role of L-T4 therapy in preventing these outcomes. Koreovaar et al. [37] analysed 47,045 pregnant women, 3.1% of whom had SCH, 2.2% isolated hypothyroxinaemia (decreased f-T4 levels with normal TSH levels), and 7.5% positive TPOAb titres, and observed a higher risk of pre-term birth. In contrast, a review by Dong et al. (38) did not find an association between SCH and RPL; in the authors’ opinion, L-T4 treatment was not supported by observational studies conducted to date. However, Cellini et al. [39] recently reported a higher risk of RPL in women with isolated high TPOAb titres and in those with positive TPOAb titres plus concurrent non-endocrine autoimmune disorders. Finally, a meta-analysis by Ge et al. [40] found an increased risk of neurodevelopmental disorders in offspring of SCH mothers, which further underlines the need for routine measurement and timely treatment of thyroid hypofunction in pregnancy. Moreover, studies published since the publication of the 2017 ATA guidelines [25] have suggested that L-T4 treatment should be undertaken before conception or early in the first trimester of pregnancy only in women with TSH >10 mIU/l and TPOAb positivity [41]. A meta-analysis by Nazarpour et al. [42] reported that L-T4 treatment in SCH pregnant women could reduce pregnancy loss. In present study, we analysed the real-world management of thyroid hypofunction, from pre-conception to post-gestational examination, in a cohort of women living in the Savona district. The analysis covers a long period in which several recommendations and guidelines were issued [19-25]. Our data suggest that the management of thyroid dysfunction has improved over time.
Few real-world studies have investigated thyroid function control in pregnant women. Sitoris et al. [43] recently performed a cross-sectional retrospective analysis of 1521 pregnant women in Belgium, who were followed up from 2013 to 2014. These authors reported a significant risk of NICU admission in newborns from mothers with positive thyroid autoimmunity and a tendency to pre-eclampsia, and low birth weight in newborns from mothers with SCH, defined as an institutional TSH level >3.7 mIU/l. In a retrospective cohort study of pregnancies with at least one TSH measurement, documented in Canada between 2014 and 2017, a total of 4417 (4.0%) pregnancies showed TSH levels >4.0–9.9 mIU/l; L-T4 (median 50 µg/day) was started either immediately (n=1808) or after confirmation of TSH levels (n=1770), but maternal and neonatal outcomes were not reported [44]. The authors stated that only 45% of treated women needed to continue L-T4 after delivery, and they hypothesized that current practice may lead to over-diagnosis and treatment of SCH [44]. Several surveys of physicians’ approaches to SCH have recently been conducted. In Israel, a survey involving endocrinologists, gynaecologists and obstetricians revealed a certain controversy; when a TSH cut-off value of 2.5 mIU/l was considered, 39% of endocrinologists advised starting L-T4, as opposed to 22% of gynaecologists/obstetricians [45]. A survey [46] conducted among Endocrine Society members after publication of the 2017 ATA guidelines [25], but to which only 10% responded, revealed that 53% had changed their practice, while 52% still endorsed a TSH level >2.5 mIU/l for the initiation/increase of L-T4 treatment. In addition, the survey revealed that responders expected only a small/very small reduction in maternal-foetal complications [46]. By contrast, in a 2018 Italian and Romanian survey, most of the 759 endocrinologists who participated were convinced of an evident association between mild thyroid impairment and adverse outcomes in pregnancy [47].
Our data showed a slight increase in gestational age from the first to the last group of women, probably as a consequence of the large interval between the first (1993) and last (2019) pregnancy monitored. No other studies of pregnancy have spanned such a long period of time. In a study by Kaski et al. [48] involving Iranian women and spanning the period 2007–2010, the median age at pregnancy was 27 years (range 15–45 years). In an Indian study [49], the women’s ages ranged from 17 to 36 years, with the majority in the age-range of 21–25 years, which was younger than in our study and, in general, in Western countries. Pre-conception BMI in our study ranged between 21 and 25 kg/m2, which is lower [48] than or similar [50] to the ranges reported in other studies. Body weight could be a determining factor in the choice of L-T4 dosage at the beginning of hormonal therapy or in the adjustment of therapy during pregnancy. In 68% of our pregnancies, L-T4 was ongoing pre-pregnancy and, despite a non-significant tendency towards less aggressive treatment in the last period (group 3), L-T4 therapy was undertaken in >94% of cases. In our group 1, a generalized 20–30% increase in L-T4 from the pre-pregnancy dosage was the initial management choice, as stated by Alexander et al. [20] and Yassa et al. [51]. According to the latter, in women with HT, who are often on an L-T4 dosage <100 µg/day before pregnancy, an adequate, one-time L-T4 adjustment can be made simply by adding two tablets per week after conception [51]. In other studies, the L-T4 dosage in pregnancy was increased according to initial TSH levels and/or TPOAb titres [48, 52, 53]. However, these studies did not consider changes b.w., which could further modulate the need for L-T4. For each 2 kg b.w. gain, we arbitrarily suggested a 25 µg/week increase over the previous dosage in the majority (>80%) of pregnancies. Our data seem to document progressively greater caution towards hyper-treatment than towards hypo-treatment (hypothyroxinaemia), as recommended in the latest guidelines [25]. However, we think that hypothyroxinaemia may be the result of underestimation of T4 in pregnancy by the current f-T4 assay, in that TT4, which is thought to be a reliable parameter [54, 55], but is not readily available in Liguria, appeared more stable in our groups during pregnancy.
Our data showed inadequate attention to iodine supplementation before pregnancy; however, an increase in the prescription of iodine supplementation during pregnancy was noted over time. To meet the iodine needs of non-pregnant, pregnant and breastfeeding women, several studies have recommended different intakes, ranging from 200 µg to 290 µg/day [6, 8, 23, 52, 55], although excessive intake (>500 µg/day) must be avoided [8, 23]. However, the available surveys have reported difficulty in routine UIC evaluation [47], and in studies in which it was evaluated, more than 50% of women showed UIC <150 µg/l. The recommendation of Negro et al. [47] to adopt a more aggressive approach to iodine supplementation seems to have been heeded in our region.
The treatment of SCH is claimed to improve maternal and neonatal outcomes. In our small number of pregnancies, however, pregnancy loss and pre-term delivery did not seem to be significantly reduced by the better control of thyroid function; neonatal weight showed minor changes among the groups. Moreover, many controversial data have emerged from observational, retrospective cohort studies and meta-analyses on the possible detrimental effect of SCH on maternal and foetal outcomes and on the role of L-T4 therapy in pregnancy. In 2017, an observational retrospective study [26] which employed the International Classification of Diseases – 9th revision – Clinical Modification codes, which are not validated for pregnancy complications, reported that the use of L-T4 in women with TSH in the range of 4–10 mIU/l was associated with a decreased risk of pregnancy loss, but that potential over-treatment was associated with an increased risk of pre-term delivery, gestational diabetes and pre-eclampsia. Two other recent meta-analyses [40, 56] found inconsistent associations between SCH in pregnancy and adverse obstetrical outcomes, while on the other hand underlining that L-T4 replacement and/or elevated f-T4 in pregnancy could increase the risks of pre-eclampsia, small for gestational age neonates, pre-term delivery, gestational diabetes and lower intellectual quotient. Finally, in a recent [57] cohort study of women with a history of RPL, no association was found between borderline SCH (TSH 2.5–4 mIU/l) or thyroid autoimmunity and RPL; treatment with L-T4 did not improve rates of pregnancy continuation past 10 weeks. However, other meta-analyses [14, 37, 42] argue in favour of L-T4 during pregnancy, in order to reduce miscarriage rates and pre-term delivery. Two cohort studies reported that L-T4 treatment might prevent pre-term delivery in TPOAb-positive pregnant women with TSH ≥4 µIU/ mL [52] and that, in GDM, maternal TSH levels >4 mIU/l seemed to be associated with an increased risk of prematurity in offspring and, albeit not statistically significantly, also with an increased risk of foetal loss, pre-eclampsia/eclampsia and low birth weight [56]. Indeed, in a real-world, single-centre study [43] of SCH pregnant women, TPOAb positivity seemed to be associated with an increased risk of NICU admission.
The strength of our study is the long period of observation, during which different approaches to SCH in pregnancy were adopted; moreover, the results cover the entire pathway from pre-pregnancy to the post-natal period. However, it also has several limitations. It was a single-centre, retrospective study involving a small number of pregnancies; the patient cohort was almost exclusively made up of Ligurian women; iodine insufficiency in the participants cannot be excluded; the sample numbers could be too small to allow rare complications of pregnancy to be examined.
In conclusion, in our region, adherence to guidelines improved over time and L-T4 over-treatment decreased. However, adherence to pre-gestational visits and iodine supplementation still need improvement. No significant differences in adverse obstetric complaints and perinatal outcomes were noted on analysing medical records collected over a long period of time. However, as recently stated by Toloza et al. [46], unresolved matters remain regarding the benefits and risks of the routine detection and treatment of maternal sub-clinical thyroid disease. In parallel with this focus on thyroid disease, future research in Italy should enhance our understanding of smaller abnormalities in maternal thyroid function, as well as thyroid autoimmunity.
Declarations
Funding
The author received no specific funding for this study.
Disclosure Statement
The author has nothing to disclose.
Ethical approval
All procedures were carried out in accordance with the ethical standards of the institution and with the 1975 Helsinki Declaration, as revised in 2008.
Informed consent
Informed consent to inclusion in the study was obtained from all patients.
Author Contributions
Massimo Giusti: study design, statistical analysis, writing
Acknowledgements
I thank Dr. Marilena Sidoti for her support in collecting Priamar medical files and Mr. Bernard Patrick for his linguistic revision of the paper.