Introduction
Premature ovarian insufficiency (POI) refers to a decrease in ovarian function before the age of 40 and it affects approximately 1% of all women 1. Estradiol is frequently decreased (below 50 pmol/L) in affected women, while gonadotropins are elevated; women report menstrual changes ranging from irregular cycles to amenorrhea 2.
In the literature, various terms are used synonymously with POI. However, the European Society for Human Reproduction and Embryology (ESHRE) Guideline as well as other international recommendations uniformly advocate the use of the term POI. This term is intended to emphasize that "premature" includes both spontaneous and iatrogenic conditions, while "insufficiency" includes the possible intermittent cessation of ovarian activity, as is frequently observed in affected women, especially shortly after the onset of symptoms 3.
The loss of primordial follicles in the ovary resulting in the development of POI may be due to genetic, autoimmune, or iatrogenic causes. In most cases, however, no cause for the loss of primordial follicles can be found. About 30% of affected women show a familial cluster.
At diagnosis, most women present with secondary amenorrhea or oligomenorrhea. Most patients report menopausal symptoms, among which subfertility is the main concern at first contact with the physician. Unlike women with a natural menopause, women with POI may still have residual ovarian function.
If POI is suspected, follicle-stimulating hormone (FSH) should be determined twice at an interval of 4-6 weeks. If the FSH value exceeds 25 U/l, POI can be diagnosed according to current ESHRE recommendations, although a variety of threshold limits are stated in other recommendations 4-7. The most commonly recommended threshold however is >40 IU/l 3. Furthermore, it is important to note that POI is not diagnosed in women without menstrual irregularities. If some menstrual bleeding still occurs, blood tests should be done on the second or third day of the menstrual cycle 3.
Menopausal complaints
Patients with POI can suffer from a variety of menopausal symptoms, just as women with natural menopause can. Typical symptoms include hot flashes, sweats, sleep disturbances, hair loss, mood swings, and decreased libido. These symptoms are due to the decrease in ovarian estradiol production; furthermore, decreased ovarian testosterone production might also lead to adverse effects on sexual desire and general well- being3,8. In the case of women with intermittent cessation of ovarian activity, symptoms can occur for a period of time and can cease when ovarian function resumes.
Systemic hormone replacement is necessary to reduce these symptoms. Furthermore, if local symptoms such as vaginal atrophy and dyspareunia are present, additional topical estrogen administration may be indicated.
Comorbidities in POI
Women diagnosed with POI are at increased risk of cardiovascular disease, leading to reduced life expectancy in affected women 9. Endothelial function is impaired in women experiencing ovarian insufficiency before the age of 45 compared with premenopausal women, but can be improved with hormone therapy 10. In addition, women with POI should be educated about other cardiovascular risk factors, such as hypertension, nicotine abuse, and obesity, and efforts should be made to minimize these modifiable risk factors.
Women with ovarian insufficiency under the age of 45 show significantly decreased bone density compared with women who experience a normal menopause 11. The risk of osteoporosis is increased in women with POI. To promote their bone health, affected women should also substitute vitamin D and calcium and engage in weight-bearing exercises in addition to receiving hormone replacement therapy (HRT) 12. HRT with physiological estrogen substitution and cyclic oral medroxyprogesterone can improve bone density in patients with POI, and after three years of therapy, bone density can be raised to the point where there is no difference between patients with POI and premenopausal women, as demonstrated by a randomized controlled trial 13. There are also studies that have shown HRT to have a more positive effect on bone density than conventional combined oral contraception using ethinyl estradiol 14, 15. Bone densitometry should be performed at diagnosis 16. If osteoporosis is present, bone densitometry should be repeated after no more than three years to allow for adjustment of the HRT if bone density continues to decline.
Women with POI might also be at increased risk of neurological disease patterns, as a neuroprotective effect of estrogen on brain function has been described 17. To date, there are no data on the effect of HRT in improving cognitive health in women with POI. Nevertheless, the ESHRE advocates HRT to reduce the potential risks to neurological health 4.
Fertility and the desire to have children
At diagnosis, infertility is usually the most impactful feature of the disease for affected women. Although approximately 25% of women still have residual ovarian function at diagnosis, natural pregnancy can be achieved in only about 5-10% of women 18,19. For infertile women with POI, there are currently no reliable treatment options to increase pregnancy rates by means of assisted reproductive techniques 20. Nevertheless, women with no desire to have children should still be informed about contraceptive methods in order to prevent an unwanted pregnancy.
Therapy
HRT is recommended in POI to control symptoms and prevent subsequent consequences of ovarian hormone insufficiency. The term hormone replacement therapy is highly appropriate, as the hormones used replace what would be present physiologically.
Hormone replacement therapy
HRT has multifactorial significance for affected patients. In contrast to hormone administration after natural menopause, POI patients are treated for true hormone deficiency. In addition to treating estrogen deprivation symptoms, the therapy is also an effective primary preventive measure against long-term risks of POI, such as cardiovascular events and osteoporosis 21.
For this purpose, HRT should be provided from the time of diagnosis until at least the age of natural menopause, and can be prescribed thereafter based on the individual wishes of the patient 20.
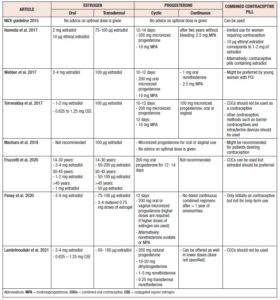
Unfortunately, there is currently no uniform standard for the optimal HRT regimen. Table 1 gives an overview of the different recommendations given by recent reviews and guidelines.
In addition to HRT, which is recommended for all women without contraindications to hormonal therapy, women with POI should substitute vitamin D3 and maintain a calcium- and vitamin D-rich diet 12 .
There are few studies that address the optimal dosage of HRT for women with POI. Although all guidelines are in agreement that HRT should be continued until the natural age of the menopause, different therapy proposals are made.
On the one hand, estrogen substitution should aim to achieve symptom control; on the other, the estrogen concentration should also be sufficient to provide effective cardiovascular protection and protection against osteoporosis.
Therefore, it seems reasonable to choose dosages that achieve physiological estrogen concentrations. Estradiol can be administered both transdermally and orally.
Three different types of estrogens are available for HRT: 17ß-estradiol, ethinyl estradiol, and conjugated equine estrogens (CEEs). 17ß-estradiol has been found in some studies to be preferable to ethinyl estradiol, due to its cardiovascular and osteoprotective effects 14, 22. CEEs are less frequently used since they have been associated with a higher risk of thrombosis, strokes and myocardial infarctions compared with 17ß-estradiol HRT in postmenopausal women23. More recently CEEs have also been linked to an increased risk of hypertension in older postmenopausal women when compared with placebo 24.
Transdermal application of estrogens avoids the hepatic first-path mechanism and achieves higher plasma levels at lower doses. In older postmenopausal women, transdermal application is associated with a lower risk of stroke, myocardial infarction, and also breast cancer compared with systemic administration 25-28. However, whether these effects translate to young women with POI is not clear4.
Guidelines and reviews in recent years recommend suggested doses ranging between 1 mg and 4 mg for oral estradiol, and 50 and 200 µg for transdermal usage 3,4,12,20,29,30. Most authors favor 2 mg estradiol for oral use or 100 µg for transdermal use as the initial dose, allowing for further adjustment over time. Only one recent review recommends adjusting doses of estradiol according to the patient’s age 20. Fruzzetti et al. suggest treating younger patients, up to 30 years of age, with high doses (50-200 µg/day for transdermal use and 2-4 mg/day for oral use). In patients aged between 30 and 45 years, the maximum dose of estradiol should not exceed 100 µg for transdermal or 2 mg for oral administration. Dose adjustment may be necessary for patients over 45, in which case the dose should be reduced to a maximum of 50 µg for transdermal use or 1 mg for oral use. The International Menopause Society (IMS) proposes adapting estradiol doses to achieve physiological levels of estradiol in a target range of 200-400 pmol/l 3.
For the treatment of local estrogen deficiency symptoms, low-dose vaginal estrogen (or prasterone) may be used in addition to systemic administration. Patients can choose between creams, vaginal rings or tablets for local application, as the efficiency of these products appears to be similar 31.
If the uterus is intact, administration of a progesterone is essential for endometrial protection. Endometrial protection is strongly recommended by all authors of recent publications, but there is wide range in terms of proposed dosages, time spans and compounds.
Some authors favor cyclic progesterone regimens, in particular shortly after diagnosis 3,12, and propose switching to a non-bleeding continuous therapy after one year3 or alternatively two years 12.
To date, there are few evidence-based data on the effects of different types of progesterone for women with POI. Micronized progesterone and medroxyprogesterone acetate (MPA) are typically used. The dose of progesterone depends on the dose of estrogen chosen and should be increased when higher doses of estrogen are applied. Progesterone can be given either continuously or cyclically for at least 10-12 days per month. If sequential administration is chosen, the dose should be higher than for continuous treatment. Most authors recommend doses of 200 mg micronized progesterone or 10 mg MPA for cyclic administration 3,4,12. Some authors also suggest endometrial protection using cyclic norethisterone3 or dihydrogesterone29. Continuous progesterone treatment is a controversial topic in the literature. Some authors do not recommend continuous treatment at all20, while others consider switching to a continuous regimen after more than one3 or two years12 of amenorrhea. Doses can then be lower in a continuous regimen, most guidelines recommending 2.5 mg MPA 4,12 or 100 mg micronized progesterone 30.
With regard to the advantages and disadvantages of the different progesterone preparations, micronized progesterone has shown more positive effects on breast cancer and metabolic risk in studies 32.
If desired by the patient, a levonorgestrel-containing intrauterine system that releases 20 µg of levonorgestrel daily can be used as an alternative. It is also important to keep in mind that HRT is not a contraceptive unless a levonorgestrel intrauterine system is used 29. Usage of transdermal natural progesterone has been shown to be inadequate in a continuous treatment to compensate for the mitogenic impact of estrogen33.
Combined oral contraceptives
The use of combined oral contraceptives (COCs) as an alternative to HRT is also controversial. COCs achieve supraphysiologic estrogen and progesterone concentrations that suppress ovulation. Since young women are generally more familiar with their use in comparison to HRT, which is more often used by older women, COCs might be preferred as a hormone therapy by some women with POI. An advantage of COCs, therefore, is that they also represent an additional contraceptive method compared with classical HRT. In most cases, ethinyl estradiol is used. However, ethinyl estradiol offers little osteoporosis and cardiovascular protection compared with estradiol, which is used in HRT 14. A randomized controlled trial showed that HRT, when compared with COCs, resulted in significantly lower blood pressure values, better renal function, and reduced renin-angiotensin-aldosterone system activity at 12 months, improving cardiovascular health in patients with POI 22. Ethinyl estradiol also shows adverse effects on lipid profile and coagulation factors and increases the risk of thromboembolic events in women with POI 3,34. Women should be administered COCs without a hormone-free interval in order to prevent menopausal symptoms associated with pill-free intervals. Continuous estrogen administration will permanently prevent symptoms of estrogen deficiency4.
When an effective contraceptive method is sought, the IMS, for example, recommends the use of COCs for two years after diagnosis 3. After this time, spontaneous pregnancy is highly unlikely because intermittent resumption of ovarian activity usually ceases during the first two years after diagnosis. At this time, a switch to conventional HRT should be made to ensure optimal protection against long-term sequelae.
Androgen substitution
In premenopausal women, testosterone is produced both in the ovaries and in the adrenal cortex. Therefore, premenopausal ovarian insufficiency results in not only an estrogen deficit but also an androgen deficiency. However, based on current studies, there is no evidence to recommend androgen therapy in women with POI 3,35. In a small cohort of women with Turner syndrome, improvement in bone density was shown with one year of methyltestosterone therapy, but in another cohort of patients with spontaneous POI, testosterone therapy administered in addition to HRT did not provide any benefit in terms of mood or quality of life36,37.
Nevertheless, patients with ongoing sexual dysfunction during HRT might benefit from androgen therapy 35.
If an androgen replacement therapy is desired, it should be applied as in postmenopausal women 35,38. It remains to be seen whether further studies will provide clearer indications for or against additional androgen therapy.
Monitoring of hormone replacement therapy
No routine tests to monitor HRT in women with POI are established today, but occasional measurement of estradiol (minimum level of 200 pmol/l) might be indicated to ensure adequate response to hormone therapy and improve lasting compliance on the part of the patient. Neither ethinyl estradiol nor estrone is determined by assays. Measurement of FSH levels is not critical for HRT verification, since a decrease in FSH levels is not frequently observed even under effective hormone therapy 39. Rather, regular follow up, e.g. annually, should take place to check the patient's satisfaction and the tolerability of the therapy 4. Breast cancer screening as well as cervical cancer screening is recommended for women with POI as for the general population 4.
Risks of hormone replacement therapy after reaching natural menopause
To date, there is no evidence for an increased risk of breast cancer from HRT before the natural age of menopause in women with POI 40.
The risk of venous thromboembolism was shown in one study to be increased in women who started HRT at less than 40 years of age compared with older women 41. If women affected by POI are at increased risk of thromboembolic events, transdermal application of estrogen should always be favored for this reason.
Hormone replacement therapy after reaching the age of natural menopause
The decision on when to stop HRT in women with POI should be made on an individual basis, depending on the patient's wishes and possible familial risk factors. Unfortunately, there are currently no data on the recommended duration of HRT in women with POI 4.
When HRT is continued at lower post-menopausal doses, it is associated with a reduction in menopausal symptoms, cardiovascular disease, osteoporosis, and type II diabetes, thus reducing overall morbidity in these patients 21,42 .
What do the guidelines say?
The ESHRE Guideline recommends cyclic combined HRT. An estrogen preparation of 17ß-estradiol with micronized natural progesterone is to be preferred. According to the ESHRE Guideline, combined cyclic HRT provides the highest endometrial protection 4, although other authors suggest greater endometrial safety by using a continuous combined regimen 43.
The current IMS white paper recommends a higher dose of estrogen than is intended for natural menopausal therapy. The goal of therapy should be to achieve physiologic estradiol levels of between 200 and 400 pmol/l 3. In any case, a progesterone preparation should be used for endometrial protection. The progesterone used can be either 200 mg of micronized progesterone for 12 days of the cycle or noretheristerone acetate or MPA. A bleeding-free continuous hormone regimen may be chosen if women have already been experiencing amenorrhea for more than 1 year at the start of therapy, or after several years of sequential therapy 3. Alternatively, the IMS recommends the use of a levonorgestrel-containing IUD for additional contraception, although studies assessing endometrial protection in women with POI are lacking. COCs containing ethinyl estradiol are inferior to HRT in terms of metabolic profile and osteoporosis protection, according to the IMS, and are recommended by the IMS only for initial therapy of POI in the first two years after diagnosis, when there is no desire to have children and safe contraception is desired.
Summary
Patients with POI benefit greatly from HRT. An HRT regimen that mimics natural ovarian hormone production and achieves physiologic estrogen levels should be chosen until the age of natural menopause. 17ß-estradiol should be preferred as the estrogen of choice and can be administered orally or transdermally. In addition, progesterone should be used if the patient has an intact uterus, preferably in a cyclic regimen for 10 to 12 days per month. Micronized progesterone, MPA and dydrogesterone are recommended. The patient should be informed that HRT is not a contraceptive and effective contraception should be discussed if necessary 44.
Studies to further characterize the optimum hormonal treatment of women suffering from POI with large numbers of included patients are needed, and patient registers, such as https://poiregistry.net, might help to aggregate a large data set and thus contribute to this effort.
HRT attenuates menopausal symptoms, maintains or increases bone density, alleviates psychological sequelae, and reduces the increased risk of cardiovascular disease.
Conflict of Interest
The authors declare that there is no conflict of interest.