Introduction
Endometriosis is a widespread, chronic, progressive disease. The main clinical symptoms of endometriosis are chronic pelvic pain and infertility, which lead to poor quality of life. On average, endometriosis is diagnosed four to eleven years after the manifestation of the first clinical symptoms [1]. There are three phenotypes of the disease: superficial peritoneal endometriosis, deep infiltrative endometriosis, and ovarian endometrioma [2]. Adenomyosis is often accompanied by endometriosis, which indicates a similarity between these pathological processes [3]. Understanding the pathogenesis of endometriosis is crucial for early diagnosis and selection of the most effective treatment regimen. Hence, further study of the pathogenesis of the disease is important, in order to be able to develop highly effective new therapeutic methods and treatment regimens.
Various factors - increased systemic and local estrogen levels, resistance to progesterone, a chronic ineffective inflammatory response, neoangiogenesis, neurogenesis, and reduced apoptosis - play a crucial role in the pathogenesis of endometriosis. Furthermore, estrogens stimulate angiogenesis, induce synthesis of inflammatory mediators, such as interleukins, and prostaglandins, and various growth factors, and increase expression of matrix metalloproteinases [4,5]. Prostaglandins and cytokines are known to be involved in the development of inflammation and are associated with infertility and chronic pelvic pain.
Oxytocin (OXT) is a promising diagnostic marker of endometriosis which can also be used in targeted therapy of the disease. To date, however, its role in the pathogenesis of endometriosis has been inadequately studied. Expression of oxytocin receptors (OXTRs) in epithelial cells and endometrial glands beyond pregnancy [6] and the expression of OXTRs and vasopressin receptors in patients with and without adenomyosis [7] have previously been demonstrated. In addition, Mechsner et al. identified myometrial cells using smooth muscle actin antibodies. In their study these authors further demonstrated that smooth muscle cells were in direct contact with the adenomyotic stroma, which showed overexpression of OXTRs compared with intact myometrium [7].
OXT is a nanopeptide hormone, which is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus and then transported to the neurohypophysis and released into peripheral blood [8]. OXT can bind to its receptor by activating the Gαq/11- protein, which in turn activates phospholipase C. Activation of phospholipase C leads to cleavage of phosphatidylinositol into inositol triphosphate and diacylglycerol [9]. In the presence of Ca2+, diacylglycerol can activate conventional and novel isoforms of protein kinase C (PKC) [10]. PKC can stimulate various signaling pathways, such as MAPK (mitogen-activated protein kinase), as well as ERK (extracellular signaling regulated kinase) and NF-kB (nuclear factor "kappa-bi"). Thus, OXT induces the expression of cyclooxygenase-2 (COX-2), stimulating the synthesis of prostaglandins by activating ERK [11,12]. Moreover, OXTR-mediated Ca2+ delivery stimulates nuclear transcription via calcineurin/NFAT (nuclear factor of activated T cells) signaling [9]. COX-2 expression is also induced by activation of the calcineurin/NFAT signaling pathway [13]. The COX-2/PGE2 (cyclooxygenase-2/prostaglandin E2) signaling pathway is involved in the pathogenesis of endometriosis. High concentrations of COX-2 lead to cell proliferation, low levels of apoptosis, cell invasion, angiogenesis, and development of the pain and infertility associated with endometriosis. The results of a study conducted by our group demonstrated a positive correlation between the level of OXT and pain intensity in women with endometriosis [14]. Local absolute and relative hyperestrogenemia is considered to be the most crucial factor in the progression of endometriosis. PGE2 is able to stimulate estrogen synthesis in endometriotic heterotopies by enhancing the transcription of genes encoding an acute steroidogenic regulatory protein and aromatase [15].
In addition, OXTR activation increases the level of Ca2+ inside the cell, by increasing the flow of extracellular Ca2+ through ion channels into the cell. An increase in intracellular Ca2+ is also necessary to stimulate the contractile activity of the myometrium. Histological assessment of endometrioid heterotopies has revealed the presence of smooth muscle cells among the stromal cells and/or macrophages that surrounded the glandular endometrial cells [16]. Additionally, contractions of smooth muscle cells mediated by OXTR activation may cause local tissue damage through induction of repair mechanisms associated with high levels of p450 aromatase expression [17].
Thus, OXTR activation in endometriosis foci may lead to overexpression of COX-2, which is accompanied by an increase in PGE2 synthesis, and hence an increase in local estradiol production.
Based on the above, OXT may play a critical role in the pathogenesis of endometriosis. Therefore, our research aimed to study the expression of OXTRs in eutopic endometrium and endometrioid heterotopies in patients with endometriosis and adenomyosis.
Methods
The study was conducted from September to December 2020. It included 123 women of reproductive age, ranging in age from 18 to 45 years. Informed consent was obtained from each patient and the women were divided into two groups: the main and the control group. The main group comprised women with surgically and histologically confirmed endometriosis of different stages, according to the revised classification of the American Fertility Society (R-AFS) (n=61), and women with adenomyosis (n=30), established on the basis of ultrasound/MRI data and confirmed by histological examination of myometrial specimens obtained through biopsy during surgery. Patients with severe comorbidities, diabetes mellitus, and/or hormonal drug intake at the time of or during the three months before the study were excluded. The control group comprised 32 patients without endometriosis based on laparoscopic evaluation. Biological samples were collected from the 2nd to the 10th day of the menstrual cycle.
Morphological study
The morphological study consisted of histological and immunohistochemical evaluation of the endometrium and of endometrioid heterotopies. Histological examination was performed according to the standard procedure. Immunohistochemical study was performed on paraffin sections with a thickness of 3 microns, which were applied to slides covered with a film of poly-L-lysine (Menzel, Germany). The Abcam Mouse and Rabbit Specific HRP Plus (ABC) Detection IHC Kit (RTU) [ab93697] was used as an imaging system (Abcam, Great Britain). A standard one-step protocol with antigen unmasking (high-temperature treatment of the tissue) in 0.01 M citrate buffer pH 6.0 was used for the immunohistochemical reaction. The immunohistochemical method included quantitative and qualitative assessment of OXTR expression using primary polyclonal rabbit antibodies to Anti-Oxytocin Receptor [ab 217212] (Abcam, Great Britain) in a standard dilution (1:200).
Digital microscopy and morphometry
Quantitative evaluation of the results of the immunohistochemical study was carried out on 905 microphotographs obtained using a microscopic image fixation system consisting of an Olympus BX46 microscope and the "CellSens 47 Entry" software. Visual fields containing tissue defects, staining defects, and artifacts were excluded from evaluation. Photography was performed at a magnification of 200-400× (eyepiece 10×, lens 20-40×), in Photo mode, exposure time 1/38 s, with maximum camera sensitivity, image size 2080×1544 pixels, and JPEG graphic image format (normal). The share of occupied expression of the studied marker on the photo was calculated using the program "VideotestMorphology 5.2" (Russia).
Statistical analysis
Statistical analysis was performed using STATISTICA software (StatSoft, Inc., version 10). Data with a normal distribution were presented as an average value ± standard deviation. The significance of differences in quantitative characteristics between the two groups was assessed using Student’s unpaired t-test (Welch correction for unequal variance). Absolute frequencies and percentages of the total number of observations were used to describe different values of the categorical data. In case of normally distributed data, the one-way ANOVA test was used to compare the expression and optical density of OXTRs depending on the degree of endometriosis prevalence. The results were considered significant at p <0.05.
Results
The average age of the patients in the main group was 32.6 ± 4.5 years, versus 31.0 ± 3.25 years in the control group. The patients in both groups were comparable for age and body mass index (kg / m2) (p>0.05). Stage I-II of endometriosis was verified in 18 patients (29.5%), and stage III-IV in 43 patients (70.5%). Adenomyosis was revealed in 30 patients.
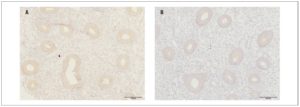
In the main group of patients, OXTR expression in eutopic endometrium (Fig. 1) in the proliferative phase of the menstrual cycle was significantly higher than expression in the endometrium of the women in the control group (55.5±8.6 and 42.1±5.9 respectively, p <0.001)
The optical density of OXTRs in the endometrium during the proliferative phase of the menstrual cycle was significantly higher in the main group than in the control group (0.09±0.01 and 0.08±0.003, respectively, p <0.05).
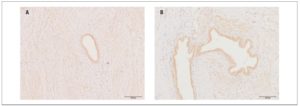
We detected no reliable differences, according to disease stage, in the expression or optical density of OXTRs in eutopic endometrium or endometrioid heterotopies (Fig.2) (p >0.05)
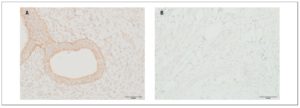
OXTR expression in myometrial samples obtained via puncture biopsy (Fig. 3) in patients with adenomyosis was statistically significantly greater than in control group myometrial samples: 58.3±21.4% versus 12.2±5.08% (p <0.05).
Discussion
Endometriosis is traditionally considered an estrogen-dependent hyperplastic and hyperproliferative disease. It is known that hormones and hormone-like substances markedly affect the proliferation and functional activity of hormone-dependent cells and trigger tissue hyperplasia. OXT works through activation of OXTRs. This study analyzed OXTR expression in eutopic endometrium and in endometrioid heterotopies (Figure 2). The encoded OXTR is a polypeptide consisting of 388 amino acids, which belongs to the family of G-protein-coupled receptors [8]. When binding to its receptor, OXT can affect the expression of COX-2 [11,12]. Activated in endometrioid stromal cells, COX-2 can further increase the production of PGE2 in steroid-converting enzymes of endometrioid heterotopies and, as a result, lead to the local production of estrogens [18,19].
Our immunohistochemical study showed expression of OXTRs in both eutopic endometrium and endometrioid heterotopies in patients with endometriosis, confirming the possibility of a direct effect of OXT on the endometrium and endometriotic foci. Furthermore, the expression and optical density of OXTRs in the endometrium of women with endometriosis were significantly higher than the values recorded in the control group.
Within the main group, no significant differences were found in the level of OXTR expression in eutopic endometrium versus endometrioid heterotopies.
Specific features of endometrioid tissue are its resistance to progesterone and pathologically high level of estradiol [20]. Progesterone resistance in endometrioid heterotopies may be associated with low expression of progesterone receptors or functional insufficiency of existing progesterone receptors [21]. An important aspect is the ability of progesterone to inhibit the binding of OXT to its receptor [22] and suppress the biosynthesis of cholesterol [23]. Moreover, estrogens, on the contrary, increase the level of OXTR mRNA [24]. Cholesterol, in turn, can increase the affinity of OXT for its receptor [8].
OXTR expression in myometrial biopsies was statistically significantly higher in patients with adenomyosis than in women belonging to the control group. Some data demonstrate that the amplitude of contractions and OXTR expression levels in myometrial smooth muscle cells are significantly higher in women with adenomyosis than in control group women. Moreover, data from the visual analog scale of dysmenorrhea positively correlate with the amplitude of contractions and level of OXTR expression [25]. Increased uterine peristalsis may be associated with its auto-traumatization. As a result, the basal endometrial cells get detached. Those cells can then either transfer upward into the pelvic cavity (endometriosis) or infiltrate into the myometrium (adenomyosis) [26].
These data indicate that OXT may represent a crucial link in the pathogenesis of endometriosis and adenomyosis. In our previous work, the high effectiveness of the OXTR antagonists was demonstrated in an experimental surgically-induced model of endometriosis. Precisely, a significant reduction in the area of endometrioid heterotopies in 68.2% cases and complete resorption of implants in 27.3% cases were achieved. These findings confirm the importance of studying the role of OXT in the pathogenesis of endometriosis. Moreover, OXTR inhibitors can be considered a promising avenue to explore with regard to the treatment of endometriosis [14].
In summary, the literature data presented, together with the results of our work, suggest that OXT plays a vital role in the pathogenesis of endometriosis. However, aspects of the implementation of this hormone in the signaling pathways involved in the development of this disease remain to be studied. Nevertheless, the use of OXTRs may become a promising direction in treating patients with endometriosis.
Disclosure Statement
The authors report that they have no conflict of interest. No funding was required for this study.