Introduction
Menopause, the absence of a menstrual period for a year, is due to a decline in estrogen and progesterone production by the ovaries [1,2]. About 60% to 80% of women experience menopause symptoms, most commonly vasomotor symptoms (VMS) and sleep disorders [3]. They can start years before, during the menopausal transition. Studies indicate that menopause symptoms can last a decade or longer, substantially affecting the quality of life of many women around their 50’s. Even though menopause hormone therapy (MHT) is the first-line effective therapy to control symptoms in menopausal women, several factors have currently decreased the use of MHT and instead suggest non-hormonal and natural approaches [4-9]. These include:
- The adherence to hormone therapy may not be satisfactory.
- There are MHT contraindications for some women.
- Women who do not wish to take hormones.
- The availability of natural products as alternatives to improve VMS.
- Women who develop VMS far from the age of menopause.
Among these natural approaches, pollen has long been used for therapeutic purposes by various civilizations. The chemical composition of pollen depends on the plant source and geographic origin, together with factors such as climatic conditions, soil type, and the activity and race of the bees. However, since pollen may be of unspecific origin (from unknown plants) and may be mixed with other bee products, standardization is difficult [10-14].
Thanks to Gôsta Carlsson, a Swedish gynecologist, in the 1940s, the external shell of the pollen which contains the allergens could be removed retaining the cytoplasm of the grain that contain numerous nutriments. The administration of these extracts could return strength and vigor to elderly patients. The process has been improved and better characterized since then. The Purified and Specific Cytoplasmic Pollen Extract known currently as PureCyTonin® formed by two extracts PI and GC Fem is safe to use since its preparation excludes allergens, and it particularly contains proteins, amino acids, sugar, minerals, vitamins, and fats. The amino acids can be identified using analytical methods. Tryptophan, a precursor of serotonin, has been documented to be present in the pollen of the plants from which PureCyTonin® is extracted. In addition, the exclusion of the shell, which constitutes a very stable protective pollen wall, makes the active compounds highly bioavailable. The production procedures are standardized and ensuring the reproducibility of the batches. High-performance liquid chromatography (HPLC) and gas chromatography can verify that the group of compounds forming the active substance is consistent between each batch. Purified and Specific Cytoplasmic Pollen Extract (PureCyTonin) is the main ingredient of finished product Serelys (Note 1).
Why use Purified and Specific Cytoplasmic Pollen Extract in menopausal women as a non-estrogenic alternative?
Several in vitro and in vivo tests have demonstrated that PureCyTonin® has no estrogenic actions:
- In the immature female rat uterotrophic bioassay no uterine growth has been observed at the high dose of 500 mg kg/day showing no estrogenic action of pollen extracts compared with ethinylestradiol. The extracts were analyzed by high-performance liquid chromatography (HPLC) and contain low, subeffective concentrations of daidzein, and genistein. Furthermore, formononetin and biochanin were not detected [15].
- In a reported gene assay using human embryonic kidney 293T cells that were co-transfected with estrogen receptors, alpha (ERa) and beta (ERb) and a luciferase reporter plasmid containing ER response elements, no estrogenic effect was demonstrated. Compared with 17-b estradiol, PureCyTonin® was not able to induce any transcriptional activation through the estrogen receptors ERs [16]. Purified and Specific Cytoplasmic Pollen Extract did not show any effect on cell proliferation in MCF7 cells up to the highest concentration tested via ER [16].
- The human breast cancer cell line MCF7 endogenously expresses estrogen receptors ERs. Data suggested that estrogens could trigger a further proliferative effect on breast cancer cells via the progesterone membrane component-1 (PGRMC1), in addition to the proliferative effect via intracellularly located receptors. Purified and Specific Cytoplasmic Pollen Extract was neutral in the cell lines alone or in combination with estradiol or growth factors on the proliferation and apoptosis, both in cells transfected either with or without the PGRMC1 [17].
- Finally, an in vitro study demonstrated that PureCyTonin® did not inhibit the CYP2D6 enzyme and thus did not interfere with tamoxifen metabolism [18].
- Concerning the mechanism of action, inhibition of serotonin uptake may play a role in the physiological action of this extract as it inhibits the uptake of [3H]-serotonin into rat cortical synaptosomes in a dose dependent manner [19]. PureCyTonin® could act as a selective serotonin reuptake (SSRI)-like therapy, potentially without the known adverse effects of SSRIs. Additionally, tryptophan, a precursor of serotonin, is present in the pollen of the plants from which PureCyTonin® is extracted. Recently, Biglia et al, in a prospective, multicenter, randomized, double-blind placebo-controlled trial evaluated the efficacy and tolerability of the cytoplasmic pollen extract in the treatment of VMS in breast cancer survivors, showing a very good safety profile [20,21]. Overall, this scientific evidence and the worldwide experience among menopausal women over 20 years shows the excellent safety profile of PureCyTonin®.
Purified and Specific Cytoplasmic Pollen Extract use in menopausal women: Efficacy and Clinical Evidence
- Good clinical results have been reported in a randomized double-blind placebo-controlled trial that evaluated the efficacy of Purified and Specific Cytoplasmic Pollen Extract after three months over the reduction of menopausal symptoms as assessed with the Menopausal Rating Scale (MRS). In addition, quality of life parameters, diaries of abnormal uterine bleeding, blood samples analysis for FSH, E2, TT, SHBG were evaluated. Indeed 65% of women responded to active PureCyTonin®, with a reduction in hot flushes compared with only 38% in the placebo group (p<0.006). The MRS evaluation revealed a 23% reduction in hot flushes in the active group compared with placebo after 2 months, and the reduction was maintained after 3 months. Improvement in tiredness, dizziness, mood, libido, headache, irritability, mood swings, and sensitiveness in the active group was higher compared with baseline (p<0.031) [22].
- In another randomized placebo-controlled trial the extract was compared with MHT. Women were randomly treated either with the active PureCyTonin® or with estrogen/progestin therapy or with placebo for 6 months and evaluated at day 0, day 90, and day 180. Results showed a notable reduction in the Kupperman index. Efficacy was slightly lower but similar to that of the estrogen/progestin therapy in peri and postmenopausal neurovegetative symptoms [23].
- A clinical trial, that included 417 women in France treated for 3 months with PureCyTonin® and evaluated with questionnaires and Visual Analogical Scales (VAS) at day 0 and day 90, showed that the frequency of hot flushes was reduced by 65% (intensity by 64%), sweating and perspiration by 66% (intensity by 67%), irritability by 54%, and fatigue by 51%. Concerning the quality of life of the women treated with the product, 324 perimenopausal or postmenopausal women were analyzed for the efficacy and tolerance of PureCyTonin®. Improvement in quality of life by 53-72% and reduction of intensity of menopausal symptoms in peri and menopausal women was observed. No differences of efficacy of the extract were observed for peri or postmenopausal women [24,25].
- In an open clinical trial, the quality of life, including sex life and reduction in hot flushes, were assessed in 50 women using PureCyTonin®. MRS and Female Sexual Functioning Inventory (FSFI) scores revealed very good efficacy with in regards to the elimination of VMS as early as after 4 weeks of initiating treatment. This effect increased during the subsequent months of treatment [26]. In a prospective, open, observational, and multicenter study not only a significant decrease of hot flushes (48.5%) was shown but also sleep disturbances (50.1%), and an improvement of mood as well [27].
- Recently, evaluation of the effect of pollen extracts in 108 menopausal women was performed showing that hot flushes, night sweats, difficulties falling asleep as well as fatigue were statistically reduced without any side effects [28].
- A comparison between pollen extracts and isoflavones on sleep disorders in postmenopausal women showed a stronger improvement of global sleep quality in the pollen treated group compared to the isoflavones group at both three (-24.7% vs -9.3%, p<0.001) and six (-52.9% vs -4.0%; p<0.001) months. In particular, the main amelioration was observed for the scores related to subjective sleep quality, sleep latency and habitual sleep efficiency. Pollen extracts achieved a better improvement in hot flushes, sleep disturbances, and menopause-related symptoms than soy isoflavones and it was most effective when the quality of sleep was the most disturbing complaint [29].
- Recently, Biglia et al. [21], performed a randomized double-blind placebo-controlled clinical trial to evaluate the efficacy and tolerability of the pollen extract in breast cancer survivors. Effectiveness was investigated through the compilation of validated questionnaires (ELIA†, MRS, PSQI, VAS), carried out one week before the initiation of treatment (T0), after 1 month (T1) and after 3 months (T2). Thirty women completed the study treated with either the pollen extract or placebo. There was a significant reduction in hot flushes in the pollen extract group after 3 months of therapy (p<0.02) (including hot flushes intensity). Even sweat discomfort, irritability and fatigue improved as assessed with the VAS scale in women of the pollen extract group (p <0.04). The total MRS score was significantly improved in both groups at 3 months, but in the pollen extract group a significant improvement was already observed after the first month (p<0.03). Regarding the ELIA questionnaire evaluation showed a significant reduction of fatigue at 3 months in the pollen extract group (p<0.0009) [21].
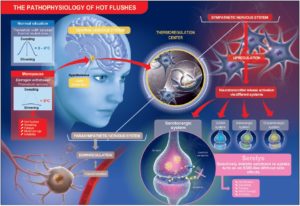
Taking these observations together, purified and specific cytoplasmic pollen extract, PureCyTonin®, shows efficacy in controlling thermoregulation, sleep, and mood in menopausal women with a safe profile. Its mechanism of action could be the maintenance of the availability of serotonin in hypothalamic serotonergic neurons, which could explain at least in part the efficacy of the extract (Figure 1).
Recommendation
As previously suggested by Genazzani et al. [30], the scientific and clinical evidence documented here support PureCyTonin® as a non-estrogenic alternative for managing menopausal symptoms including VMS and the possible use in cancer survivors. Therefore, PureCyTonin® and the finished product, could be recommended as a non-hormonal alternative for the management of menopausal symptoms and to improve patients’ quality of life [30].
Conclusion
PureCyTonin® is an effective and safe non-hormonal alternative for alleviating menopausal symptoms in women with contraindications to MHT or in those not wishing to use hormones. PureCyTonin® has proven to be safe and effective, improving hot flushes, sleep disturbance, and quality of life in menopausal women.
Declaration of interest
There is no conflict of interest to declare.