Introduction
Infertility has recently become a major health problem, affecting 15-30% of the reproductive age population [1]. Diseases and conditions that may damage women's reproductive system and cause infertility include: abnormalities of uterine development, genital system inflammation, previous gynecological surgery, endometriosis, fibroids, endometrial and cervical polyps, and various environmental factors and harmful lifestyle habits [2-8].
Tubal factor infertility is generally due to tubal obstruction, distortion or intrinsic dysfunction of the epithelium [9]. The most prevalent cause of tubal factor infertility is pelvic inflammatory disease (PID) causing tubal impairment, not only by producing macroscopic structural distortions [10] but also by affecting directly the tubal epithelium [11,12]. The concept that the fallopian tubes have their own microbiota, similar to other female genital tract districts, is a recent acquaintance [13,14]. However, this aspect has been marginally evaluated only in studies with a small cohort of patients and with important methodological biases [15-17].
This short review aims to present the most recent evidence on the possible role of tubal microbiota on female infertility, focusing on its potential diagnostic effectiveness and, in particular, on the role of hysteroscopy.
Female infertility: what is the real role of the tubal factor?
Infertility is currently recognized as a worldwide health issue and has been classified by the World Health Organization (WHO) as a civilization disease [1]. Its incidence is increasing year by year, and it is now estimated that a total of 10-12% of women is infertile [18,19]. A serious problem is the decreasing female fertility, which mainly results from the raising of the age limit in the decision to have a child. The main cause of age-related fertility decline is the increase in genetic abnormalities in aging oocytes [20]. This increases the miscarriage rate and the risk of birth defects in the offspring. Female fertility already declines slightly around age 20, and a significant decline is seen around age 35 as the number of primary follicles in the ovaries rapidly decreases. The risk of a diagnosed or undiagnosed miscarriage is estimated to be about 75% in women over 40, while before the age of 30 it is no more than 7-15% [21].
Tubal factor infertility is currently the most common cause of female infertility and is diagnosed in up to 35% of patients [22]. It generally occurs secondary to tubal obstruction, distortion, or intrinsic dysfunction of the epithelium [9]. Factors related to tubal infertility include occlusion of the distal fallopian tube in 85% of cases [22]. Occlusion may be the result of various inflammatory conditions such as pelvic inflammatory disease (PID), endometriosis, and previous abdominal or pelvic surgery9. Furthermore, this type of infertility can be caused by any intra-abdominal pathological process that leads to the formation of adhesions. The most prevalent cause of tubal factor infertility is PID and acute salpingitis caused by several pathogens. PID affects tubal patency not only with macroscopic structural distortions [10] but also directly affecting the tubal epithelium [11,12]. Several cytological studies have demonstrated that, common and uncommon PID pathogens (Chlamydia trachomatis, Neisseria gonorrhoeae, Escherichia coli, Mycoplasma hominis, Mobiluncus, Bacteroides and Ureolyticus) cause ultrastructural changes consisting of sloughing and/or destroying of ciliated cells, with subsequent cessation of ciliary activity, disruption of cell junctions and apoptosis of epithelial cells. These are the consequences of several pathogenic mechanisms such as direct cytotoxic effect, immune response, secretion of chemokines and cytokines [23,24]. Most studies have confirmed an association between the number of episodes of PID and the risk of infertility - therefore, a woman with 3 PID episodes has a risk of remaining infertile in up to 75% [25]. Several seroepidemiological studies have demonstrated the effects of Chlamydia trachomatis and Neisseria gonorrhoeae on fallopian tube damage and subsequent infertility [26-28]. These epidemiological studies have shown that women with laparoscopically or hysterosalpingographically confirmed tubal infertility factor have a significantly increased seroprevalence of Neisseria gonorrhoeae compared to fertile women. However, there is an evident decline in the incidence of Neisseria gonorrhoeae and Chlamydia trachomatis [25]. For instance, during the 1980s, the incidence of Neisseria gonorrhoeae in women with PID was between 40-50%, while recent research has shown that this incidence is now up to 20% in women with PID [25]. On the other hand, there is limited evidence in the medical literature that other sexually transmitted pathogens, including Mycoplasma genitalium, Trichomonas vaginalis, and other microorganisms, within the vaginal microbiota may be important factors involved in the female infertility [25]. Given that there is a parallel increase in the incidence of tubal infertility, which today is up to 35%, it is clear that understanding the impact of microbiota disorders on fertility could help solve this problem.
Endometriosis shows a similar effect on fallopian tube function [29-32]. Although the pathophysiology of endometriosis is not fully understood, the most widely accepted theory is retrograde menstruation of debris from the uterus through the fallopian tubes attaching to the peritoneal surface [33]. Ectopic endometrium shows a tendency to chronic inflammation by producing reactive cytokines and chemokines, resulting in tissue scarring and therefore disrupting the fallopian tube function [5]. Among the women suffering from endometriosis, tubal infertility factor may be present in up to 15% [5]. Several other causes of tubal infertility may include scarring after abdominal and pelvic surgery, fibroids near the tubal ostium, and pelvic tuberculosis, which occurs in nearly 20% of patients with pulmonary tuberculosis [34].
Microbiota and female fertility: is there also a connection with the tubal factor?
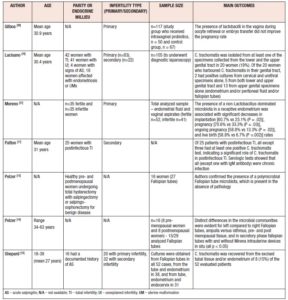
There is a growing body of evidence related to the importance of the urogenital microbiota associated to reproductive outcomes, both for achieving pregnancy naturally or using ART, as shown in Table 1 [13,35-38]. Nevertheless, although some data advocate a significant relation between alterations in the urogenital microbiota and infertility, the overall evidence is conflicting. In women with idiopathic infertility and in those who have undergone repeated unsuccessful IVF cycles, anaerobic bacteria have been shown to predominate and Lactobacilli spp. are less abundant, especially Lactobacillus crispatus and Lactobacillus iners [35]. On the other hand, a randomized controlled trial examining colonization and pregnancy rates in 117 women taking intravaginal probiotics showed that the presence of Lactobacilli spp. in the vagina during oocyte retrieval or embryo transfer did not improve pregnancy rates [39]. Although the vaginal microbiota is the most extensively studied, namely in terms of vaginal microbiota restoration strategies and even in terms of vaginal microbiota transplants, recent research has also focused on other regions of the urogenital tract, primarily the endometrium. In a study by Moreno et al., the presence of a non-Lactobacillus-dominated microbiota in a receptive endometrium was associated with significant decreases in implantation [60.7% vs 23.1% (p=0.002)], pregnancy [70.6% vs 33.3% (p=0.002)], ongoing pregnancy [58.8% vs 13.3% (p=0.002)], and live birth [58.8% vs 6.7% (p=0.0002)] rates [37]. Furthermore, they have detected different bacterial communities from paired endometrial fluid and vaginal aspirate samples within the same individuals, indicating that the vagina does not accurately reflect microbiota in the endometrium [37]. Another study demonstrating the importance of the endometrial microbiota for IVF success and overall female fertility has shown that samples positive for Streptococcus viridans were predictive of very low birth rates (p=0.04) whereas Lactobacillus spp. linked with a live birth rate in up to almost 90% [40]. Similar to the vaginal microbiome, there is also conflicting evidence of the role of the endometrial microbiota in female infertility. In a case-control study examining 28 patients with repeated implantation failure and 18 healthy controls, no significant association was found between endometrial Lactobacillus dominance and infertility [41]. Another study that extends the knowledge of female idiopathic infertility compared the vaginal microbiota of infertile women affected by different clinical/physiological conditions with that of healthy and bacterial vaginosis affected women [42]. Their analysis revealed that Lactobacillus crispatus, Lactobacillus gasseri and Lactobacillus iners distinguished idiopathic infertile women from the other groups [42].
The role of the vaginal and endometrial microbiota in potential infertility can be applied to the tubal milieu, which is currently a hot topic in clinical research. The concept that the Fallopian tubes have their own microbiota, similar to that of other female genital tract districts, is a recent acquaintance [13,14]. Several microbial communities reside in the Fallopian tubes even in absence of infection [14,43]. Members of the Firmicutes phylum, such as Staphylococcus spp., Enterococcus spp., and lactobacilli, as well as Pseudomonadaceae, including Pseudomonas sp. and Burkholderia sp., along with species belonging to both Propionibacterium and Prevotella genera, have been reported as the predominant taxa of the Fallopian tube microbiota [14,44]. Given the importance of the tubal epithelium in the conception process, tubal microbiota should play a fundamental role in warding off infections and in maintaining a favorable environment. Despite copious evidence of the favorable association between an adequate site-specific microbiota and fertility in the other districts of the female reproductive system [45,46], only few studies have been focused on the characterization of the tubal microbiota. This is owing to the technical difficulties in sampling retrieval [47]. Several studies have confirmed that tubal microbiota has an important role in maintaining a normal reproductive function [13,48]. The microbiota has a direct effect on tubal lumen and protects it by opposing pathogenic microbes. Also, it has been determined that a difference in tubal microbiota composition in the right Fallopian tube compared to the left can be explained by hormonal influence on tubal microbiota composition [14,48]. Although there is some research showing that microbiota plays an important role in the reproductive endocrine system by interacting with estrogen, androgens, insulin and other hormones, future research is needed to explain the insight of underlined molecular pathways. As shown in Table I, there are studies that have compared tubal and urogenital microbiota with subject’s age, parity, and type of infertility (primary or secondary). Due to the demonstrated differences in the vaginal and endometrial microbiota and their interrelationships in the evaluation of infertility, further studies are needed to determine the differences between the vaginal, uterine cavity, and fallopian tube microbiota in the same individual with unexplained infertility. Moreover, a versatile nature of the tubal microbiota should be considered not just as a potential cause of female infertility, but also as a potential source of microbial seeding in postsurgical infection [14].
Another potential clinical consideration to take into account is the importance of simultaneously investigating the male partner to obtain a better interpretation of the female microbiota. It is well known that male dysbiosis impairs sperm motility, morphology and concentration. Males with low quality sperm morphology display increased levels of Ureaplasma, Mycoplasma, Enterococcus and Prevotella in comparison to normal males which showed a dominant presence of Lactobacillus [49,50]. The interactions between female and male genital tract microbiota during sexual intercourse have emphasized the possible effect of the microbiota on the reproductive function [51]. However, despite a possible link between pathogens and male infertility, further studies are needed to determine the role of seminal microbiota in fertile and infertile subjects.
Hysteroscopy: a possible keystone in understanding tubal microbiota
Despite the reported favorable association between an adequate site-specific microbiota and fertility in the other organs of the female reproductive system [45,46], only a few studies have investigated the role of tubal microbiota. The majority of the available data come from studies obtained by laparoscopic access, specifically, by salpingectomy [16,17] or by biopsies of the distal portion of the fallopian tube [15,17].
Furthermore, in these studies, tubal microbiota has been mostly assessed collaterally to the detection of pathogens. A major flaw of currently available data is that the surgical excision of the tubes represents a methodological bias for cytological and microbes sampling. First, the mechanical manipulation and the electrocoagulation may impair the microbiological and cytological content. Furthermore, salpingectomy reduces the area of analysis only to a restricted portion of the tube, without considering the microbial population present in the entire tubal lumen. Another important aspect is that salpingectomy does not ensure the anatomical and functional integrity of the inner female genital tract.
Taking into account the above-mentioned limitations, the application of non-invasive techniques in clinical and scientific settings is mandatory. From this standpoint, hysteroscopy may be the method of choice for the indirect evaluation of the tubal integrity. Currently, hysteroscopy is considered an ideal diagnostic procedure for the evaluation of the vaginal walls, cervical canal, uterine cavity, endometrium, and fallopian ostia [52]. Outpatient hysteroscopy has obvious major benefits for the patient and diagnostic procedures can be performed without anesthesia using modern hysteroscopes with a 5-french surgical channel that allows a direct view of the structures without compromising their integrity [53]. This approach was used by some authors to obtain tubal tissue samples to clarify etiology of salpingitis using a cytobrush inserted through the working channel [54]. The advantages over the laparoscopic approach are minimal invasiveness and lower financial burden, indicating suitability in clinical and scientific settings. If one also considers methodological aspects in the determination of the microbiota, hysteroscopy is the most practicable of the currently available methods. However, the use of the cytobrush has several pitfalls that should be underlined. Cytobrush can cause mechanical trauma and potentially affect future fertility, and because of its limited flexibility and great diameter, cytobrush is not quite acceptable for microbiological and cytological sampling. Although hysteroscopy appears to be the most appropriate minimally invasive method for tubal factor testing, there are currently few and conflicting data in the literature supporting its use. For this reason, further studies are needed to better clarify this topic, especially in patients who are electing to undergo IVF/ICSI.
Conclusions
To date, there is currently not enough high-quality and conclusive research regarding tubal factor infertility and the tubal microbiota, and thus no conclusions can be made as to the possible causes of tubal infertility in women who do not have a clear pathological substrate. Given that the incidence of tubal infertility factor is constantly increasing, and the incidence of previously known infectious causes is declining, it is extremely important to encourage research to identify the real composition of the tubal microbiota. On the other hand, the potential importance of the role of hysteroscopy in elucidating tubal infertility factor is currently underestimated. Current diagnostic methods do not show good enough results given the evident increase in the proportion of unexplained causes of tubal infertility, so it is necessary to give the opportunity to all the advantages that hysteroscopy offers - minimal invasiveness, outpatient setting, reproducibility and he ability to “see and treat”. Another key point is to understand the tubal microbiota in relation to some demographic variables such as ethnicity or parity and the possibility to study microbiota in tubal infertility with different etiologies. Thus, large, multicenter, well-designed studies, potentially using hysteroscopic sampling methods are needed to clarify the relationship between tubal microbiota and female infertility.
Declaration of Interest: The authors report no conflicts of interest.