Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinically reversible neurotoxic condition characterized by loss of consciousness, visual disturbances, seizures, headaches, confusion, vomiting, and cortical blindness [1]. PRES can be caused by several clinical conditions, including immunosuppression, chemotherapy, hypertension, renal damage, and infection [1-4].
The incidence of PRES is low, and the condition is predominantly observed in female patients [4]. In obstetrics, PRES is a well-known consequence of eclampsia. Typical neuroimaging manifestations of PRES include vasogenic edema, predominantly in the parieto-occipital lobes [2,4]. The prognosis of PRES is favorable and, in most patients, clinical symptoms and imaging lesions are reversible [1-5].
Uterine fibroids are a common cause of anemia, dysmenorrhea, and heavy menstrual bleeding (HMB). Gonadotropin-releasing hormone antagonists (GnRH-a) decrease fibroid size; hence, GnRH-a therapy can be used for this condition [6].
There are some reports regarding PRES after correction of severe anemia [4,7,8]. However, the relationship between improvement of severe anemia and PRES is not yet clear. There are a few reports of PRES associated with GnRH agonists [9,10]; however, there are no reports of PRES related to GnRH-a.
Here, we report a case of PRES that developed secondary to GnRH-a use after treatment of HMB-induced severe anemia caused by a large uterine fibroid.
Case presentation
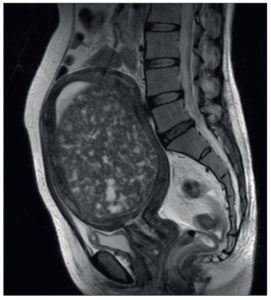
A 52-year-old woman was referred to us with a benign uterine tumor and HMB. The patient had a body mass index of 21 kg/m2 and was gravida 3, para 2, ectopic pregnancy 1. She had severe iron deficiency anemia (blood hemoglobin, 5.4 g/dL) and a pelvic magnetic resonance imaging (MRI) that revealed a uterine fibroid extending toward the height of her navel (Figure 1). MRI and endometrial tissue diagnosis reported the absence of malignancy.
Treatment with of oral iron preparations (200 mg daily) for 40 days successfully raised her hemoglobin level to 11.9 g/dL. The patient preferred hysterectomy for treatment of the fibroid and HMB. In preparation for the surgery, she was given a 40 mg daily dose of relugolix, a GnRH-a, to decrease fibroid size and prevent HMB until the day of the surgery.
Eight days after starting GnRH-a therapy, the patient was brought to our hospital’s emergency room after having a seizure while driving. She had a blood pressure of 185/116 mmHg and a Glasgow Coma Scale (GCS) score of E4V1M1; therefore, she was admitted to the intensive care unit and was supported with artificial ventilation. We administered calcium channel blockers and anticonvulsant drugs because the patient continued to have convulsions.
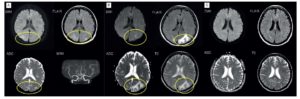
Damage to the brain, hemorrhage, viral infection, collagen disease, and electrolyte imbalance were ruled out. Cerebrospinal fluid analysis revealed no abnormalities. The brain MRI on the first day of admission showed no cerebral infarction; however, it showed lesions in the parietal and occipital lobes. The following findings were observed: low-to-equal signal on diffusion-weighted imaging (DWI); high signal on fluid-attenuated inversion recovery (FLAIR); and high signal on the apparent diffusion coefficient (ADC) map. No obvious cerebral infarction was shown by magnetic resonance angiography (Figure 2A). The MRI findings became more prominent on day four of hospitalization. Since the radiologic findings were consistent with the presentation of PRES, this condition was diagnosed as the cause of the patient’s seizures (Figure 2B). Relugolix was discontinued on the day of hospitalization and was noted as a possible trigger of PRES. We continued treatment with anticonvulsants and antihypertensive drugs.
The patient’s condition improved and two days after admission she was removed of tracheal intubation. On day five, the patient’s GCS score was E4V5M6 and visual disturbances reported upon admission, such as seeing rainbows around lights and hallucination of numbers on faces, improved. Overall, PRES symptoms gradually decreased without recurrence, and MRI findings slowly normalized. The patient was discharged 22 days after hospitalization. Anticonvulsant treatment was discontinued to avoid drug-induced liver injury, and a follow-up MRI performed 92 days after PRES diagnosis revealed that all prior abnormal findings had completely disappeared (Figure 2C).
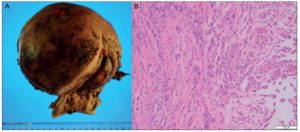
Hysterectomy was performed 99 days after PRES diagnosis. The hysterectomy was initiated laparoscopically; however, due to the size of the uterine fibroid, the procedure was changed to abdominal surgery, allowing the removal of a 2,020 g uterus (Figure 3A). Histologically, spindle-shaped smooth muscle cells without atypia were observed, confirming the diagnosis of uterine myoma (Figure 3B). The patient was discharged 7 days post-hysterectomy and to date has had no sequela related to the PRES.
Discussion
Currently, the mechanisms underlying the etiopathogenesis of PRES are unclear. PRES is a disorder of dysregulated perfusion, which leads to reversible vasogenic edema. There are several theories explaining how vasogenic edema occurs. According to the “hypertension and hyperperfusion theory,” severe hypertensive injury to the capillary bed and the breakdown of the blood-brain barrier, result from increased cerebral perfusion pressure, leading to extravasation of plasma. Another possible mechanism is the vasospasm of cerebral vessels, which leads to hypoperfusion of the brain parenchyma. Brain ischemia leads to cytotoxic edema with or without actual cerebral infarction [11-15]. Endothelial dysfunction has also been implicated in PRES pathogenia. Cytokine release may be related to a systematic condition, such as eclampsia, chemotherapy, immunosuppressive medications, sepsis, or autoimmune disease causes endothelial injury. Release of vasoactive agents increases vascular permeability and edema formation [2,12,13]. It is believed that PRES may be an outcome of multiple factors rather than a single factor.
In our case, we postulate that two factors were involved in the onset of PRES, namely, chronic anemia and its correction and hypertension and endothelial injury due to iatrogenic menopause caused by GnRH-a therapy. Chronic anemia may result in compensatory cerebral vasodilation. Anemia correction leads to increased blood flow and increased blood viscosity. A rapid increase in hematocrit levels elevates blood viscosity and vascular resistance and induces acute vascular endothelium dysfunction. This leads to endothelial damage and extra-vascular leakage [5,12]. There is evidence regarding PRES after blood transfer for severe anemia [4,7,8]. In the present case, anemia was gradually corrected solely with oral iron preparations, which took 40 days.
Despite one report of PRES being caused solely by iron preparations, this is not a common occurrence [16]. Hence, we postulate that in our case iatrogenic menopause was another causative factor in the development of PRES.
GnRH agonists and antagonists artificially induce menopause. Menopause can cause hypertension because of decreased estrogen [17], which can lead to increased endothelial injury.18 Furthermore, studies show that low estrogen levels affect vascular resistance [17,18]. Therefore, it is likely in our case that correction of chronic anemia and iatrogenic menopause-induced hypertension, endothelial damage, and hyperperfusion were causal factors of PRES. However, it is crucial to note that most cases of elevated cerebral blood flow do not result in PRES, even when patients are affected by the same degree of anemia [9]. We speculate that this could be due to individual differences in the blood-brain barrier [16].
As demonstrated in our case study, brain MRIs are extremely helpful for the diagnosis of PRES. Typically, T2-weighted MRI images of patients with PRES show symmetrical white matter hyper-intensity in the occipital and parietal lobes [2,19]. Additionally, because PRES involves cerebrovascular edema, DWI’s show low-to-equal signals, whereas ADC maps have high signals [2]. Similar findings were observed in our case (Figures 2A and B). The parieto-occipital region has been observed in 98% of PRES cases [20]. This is because the adrenergic sympathetic innervation is adequately supplied to vessels of the carotid, compared to that of the vertebra-basilar vessels. Thus, we believe that cerebral vascular autoregulation is likely to be dysfunctional, and PRES is likely to appear in the parieto-occipital lobes [11].
Owning to the lack of specific treatments for PRES, clinicians rely on symptomatic treatment, focusing on reducing both hypertension and convulsions and discontinuing the causative drug.2,5 In our case, the administration of antihypertensive and anticonvulsant drugs improved PRES symptoms without sequelae. Overall, PRES has a good prognosis [1,5,11,12,19]. However, some cases of severe functional impairment and mortality have been reported [21,22].
Case reports have limitations in clarifying the relationship between PRES and GnRH-a. In fact, it is unclear from this case whether vascular damage was caused directly by GnRH-a or indirectly by hypertension secondary to lower estrogen levels. More reports describing PRES during correction of anemia with GnRH-a therapy are needed.
In conclusion, PRES may be caused by several factors. In the present case, the correction of chronic anemia and iatrogenic menopause due to the use of GnRH-a may have caused PRES.
Author contributions: Each author mentioned on the title page has significantly contributed to the creation of this manuscript, and no individuals qualifying for authorship have been omitted.
Ethics statement: This work was undertaken in accordance with the tenets of the Declaration of Helsinki. Informed written consent for publication of this case report was obtained from the patient.
Acknowledgments: This case report did not receive funding of any kind.
Conflicts of Interest Statement: The authors declare having no conflicts of interest.