Introduction
Aging, socio and economic factors, health status (e.g. -- cardiovascular diseases), vasomotor symptoms (VMS), hormonal variation, and psychiatric illness (depression and anxiety), have been identified as potential causes of sleep disorders during the menopausal transition (perimenopause), menopause and postmenopause [1-8]. VMS such as hot flushes (HF)/sweating affect 80% of women during the peri- and postmenopause [9]. HF are characterized as a loss of heat as consequence of estrogen driven changes in neurotransmitters, causing thermoregulatory instabilities at the hypothalamus [10-12]. There is also peripheral vasodilatation, that leads to increases in sweating and chills, that could be accompanied by anxiety [13]. There is evidence showing how HF are concerning up to 80% of women attending health care providers, indicating that 30% of women exhibited moderate and severe HF lasting from 1 to 2 years up to 10 years [14]. These studies also report that the severity and frequency of HF are higher in women with prior poor psychological (e.g., stress), and health status (e.g., obesity and cardiovascular diseases). Furthermore, the severity of HF is also dependent on dietary and other habits (e.g., smoking), that could concomitantly cause sleep disorders during the menopause transition and postmenopause [15,16].
Sleep disorders like insomnia, obstructive sleep apnea (OSA), and restless legs syndrome (RLS, also known as Willis-Ekbom Disease), could increase among perimenopausal and postmenopausal women [17-20]. All of these disorders lead to occupational, cognitive, psychological, behavioral, and physical distress in women, affecting both their wellbeing and quality of life [21-25]. Furthermore, these sleep disorders can cause work accidents, absenteeism, and low performance [26,27]. Additional symptoms affecting women’s quality of life associated with sleep disorders include muscle pain, headaches, sexual and genitourinary malfunction [14,28-30]. Several lines of evidence point out a need of having reliable treatments that simultaneously ameliorate VMS and sleep disorders in women transitioning to menopause and experiencing the postmenopause [31]. Menopausal Hormone Therapy (MHT) is a general treatment for all symptoms related to the menopause. However, for women with estrogen dependent diseases and thromboembolic events, MHT is contraindicated, suggesting the need of both developing and implementing alternative treatments. These additional options are also needed for those women who do not want to take hormones during their different menopausal stages. Therefore, this review reports scientific evidence regarding the use of alternative options to MHT that ameliorate sleep disorders caused by hormonal variation. Particularly this review is focused on the possible association between VMS and sleep disorders to identify and propose non-hormonal/non-pharmacological alternatives for simultaneously treating these symptoms in peri- and postmenopausal women who have contraindication for MHT use or those who don’t wish to take hormones.
Epidemiology, prevalence, and incidence of sleep disorders during menopause
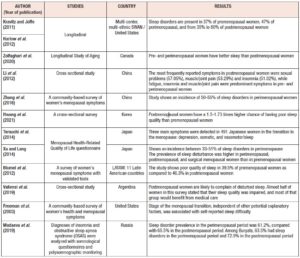
The Study of Women's Health Across the Nation (SWAN) reports sleep disorders in 37% of premenopausal women, 47% of perimenopausal, and from 35% to 60% of postmenopausal women [13]. Similar results have been reported in another longitudinal study performed in Canada, which shows how pre and perimenopausal women have better self-reporting sleep quality than postmenopausal ones. Specifically, postmenopausal women exhibit a higher risk of presenting either insomnia or OSA, than pre- or perimenopausal ones. This study also shows that there is not an apparent association between menopausal status and the RLS [8] ). Additional supportive evidence comes from multiethnic cross-sectional studies, showing that women during the menopausal transition and postmenopause exhibit higher risks of having sleep disturbances than non-menopausal women [33]. For instance, Li et al. [34] and Zhang et al. [35] show that Chinese women exhibit an incidence of sleep disorders between 50% to 55% during the perimenopause. Additionally, a study in Korea shows that both peri- and postmenopausal women have between 1.5 to 1.73 times higher chance of having poor sleep quality than premenopausal ones [36]. Japanese perimenopausal women exhibit sleep disorders within a range of incidence between 33% to 51% [37,38]. Likewise, and according to the Pittsburg Sleep Quality Index (PSQI), 39.5% of premenopausal women and 46.3% of postmenopausal Latin American surveyed women, exhibit poor sleep quality, which intensify the severity of menopausal symptoms [39]. Similar results were obtained from Argentinian women showing that 46.7% of postmenopausal women presented poor sleep quality [40]. Kravitz et al. [41] report that the incidence of poor sleeping in Caucasian women is 40%. Likewise, Madaeva et al. [42] report that European – Russian women exhibit a sleep disorder prevalence of 61.2% and 65.5% for peri- and postmenopausal women respectively. Furthermore, the latter authors also report that women belonging to the ethnic group Buryats, show a prevalence of sleep disorders in 63.5% and 72.9% of pre- and postmenopausal women, respectively [42]. Overall, these studies also report that women exhibiting sleep disorders are prone to presenting severe cardiovascular diseases and depression, which reduce women’s quality of life and quotidian performance. Therefore, both longitudinal and cross-sectional studies report multifactorial evidence showing that sleep disorders during the menopause transition and post menopause are related to hormone levels, affecting women independently of their ethnicities (Table I). These lines of evidence also emphasize that menopause related hormonal changes are possibly affecting both women’s thermoregulatory physiology and sleep quality simultaneously. These evidences here presented indicate the need to develop integral interventions/treatments that act concomitantly on VMS and sleep disorders among affected women at any menopausal stage.
Sleep disorders are influenced by hormonal variation in menopausal women
Hormonal changes influence sleep cycles. There is a significant correlation between hormonal status and sleep quality indicating that ovarian / sexual hormones regulate women’s sleep [43]. For example, during menstrual cycles, there are recurrent changes in production of estradiol (E2), progesterone, luteinizing hormone, follicle stimulating hormone (FSH), prolactin, and growth hormone, altering sleep quality and circadian rhythms [25,44]. Similarly, during the perimenopause, research based on polysomnographic studies, demonstrates how high levels of FSH are linked to sleep architecture. Scientific evidence shows how increases in FSH is positively associated with wakefulness after sleep onset and negatively associated with slow wave sleep [14,45]. Furthermore, data from the SWAN longitudinal study with 8 years of following up of a cohort of more than 3,000 multiethnic women, show that women during their early menopausal transition, and postmenopausal stage exhibit significant difficulties in falling sleep, waking up several times during night, and waking up early [46]. Supporting odd ratio values, from a polysomnogram based study, were significantly associated with decreases in E2 and increases in FSH. In addition, it has been reported that low levels of estrogen, alterations in E2 to total testosterone ratio, can lead to insomnia in perimenopausal women, causing anxiety, depression, or hypertension [47-49]. Moreover, in both peri- and postmenopausal women, there are decreases in both E2 and melatonin levels that exacerbate the difficulties of falling asleep and waking up several times [32]. Concerning to the role of progesterone in sleep quality, there is evidence showing that micronized progesterone treatment, can improve sleep disorders in postmenopausal women [50], suggesting the possible role of this hormone in women’s sleep patterns. Other sleep problems such as OSA in postmenopausal women might be caused by the loss of the protective effects of either estrogen and/or progesterone. Likewise, it has been demonstrated that reductions in progesterone levels can promote both fat redistribution and increases in neck circumference, fostering aerial obstructions among postmenopausal women affected with OSA [8]. On the other hand, RLS and its association with hormone levels is still a matter of research. Polo-Kantola et al. [51] reported among asymptomatic postmenopausal women, the independence between RLS and both E2 and FSH levels. This study suggests that pathophysiology of the RLS is more related to aging than hormone levels. RLS pathophysiology is linked with both iron deficiency and decreases in dopamine, with levels that tend to drop with aging. The later indicates that not only postmenopausal women are prone to exhibit higher prevalence of RLS, but also the role of neurotransmitters regulating sleep-wake dynamics [52-54]. Overall, these facts allude that variation in the secretion of sexual hormones induces sleep disorders during the perimenopause, and the postmenopause. This apparent relationship has been a key factor for developing research to propose treatments such as MHT for enhancing women’s quality of life.
Vasomotor symptoms might be associated with sleep disorders
Insomnia is the commonest reported sleep disorder during the menopause and its transition [8,30,32,55-58]. Moreover, insomnia is considered as a secondary consequence of VMS, including HF, night sweats, and palpitations, which are in relation to estrogen levels [59-61]. For instance, Campbell and Murphy [62] have shown how HF cause insomnia in 29% of women [62]. Similarly, the SWAN study indicates that women with moderate to severe HF exhibit up to 3 times more probabilities of presenting sleep disorders (e.g., night awakenings and insomnia), than women without HF [32]. Furthermore, HF and sweating are statistically related to depression, and anxiety, which also lead to insomnia and vice versa [5,27,31,63-66]. On the other hand, symptoms associated to sleep apnea, in postmenopausal women, include night sweats, daytime sleepiness, and headaches [67], indicating a possible association between VMS and OSA. For example, intermediate, and severe OSA, could be related to severe VMS in perimenopausal women with hypertension and a body mass index of less than 25 kg/m2 [68]. This report advocates how thermoregulatory neuronal control, associated with HF, are possibly working together with both respiration and sleep hormonal regulators such as progesterone, estrogen, and FSH [12,69]. Moreover, low levels of progesterone increase fat accumulation and distribution reducing breathing capabilities in menopausal women [8]. In addition, women treated with MHT for the alleviation of HF, reduced their sleep disorder complaints associated with OSA and RLS. However, there is not sufficient data to establish a strong and direct cause - effect relationship between OSA or RLS and VMS. A study reporting the causes of sleep disorders in peri- and postmenopausal women shows independence between RLS and VMS [1]. This array of evidence suggests that both sleep disorders and VMS could co-exist, and are each other secondarily linked to sexual hormone withdrawal such as estrogen and progesterone and variations in FSH and melatonin.
Treatments for VMS and their concomitant sleep disorders
Pharmacological hormonal interventions
MHT is the first prescribed therapy for VMS, and it is prescribed for managing sleep disorders during the different stages of the menopause. Particularly this intervention, has shown efficacy by increasing sleep quality among women experiencing VMS exclusively [70,71]. However, there are reports of women presenting adverse effects and consequently non compliances leading to refusing MHT and turning to non-hormonal treatments as a “natural” alternative [72,73]. Several guidelines recommend MHT for both short periods of time and low doses. Nevertheless, since the first publication in 2002 of the US clinical trial of the Women’s Health Initiative (WHI) Study, the number of MHT prescriptions has dropped 60% in European populations, by 46% in the USA and by 28% in Canada [74-76]. In Asian populations such as Korea, over 70% of women were prescribed MHT for less than 1 year [77].
One alternative to pharmacological interventions are phytoestrogen supplements, which are found in soybeans (isoflavones), in Humulus lupulus, flaxseed (lignans), fruits, vegetables and whole grains [78,79]. Isoflavone based therapy has shown efficacy in reducing the severity of HF [78-85]. There is evidence showing no correlation between isoflavones’ dosage and the severity and frequency of HF [86]. Regarding sleep disorders, isoflavone interventions in Asian populations have shown positive results increasing both sleep quality and duration during the peri- and postmenopause [84,85]. However, there are reports showing no correlation or negatively affecting sleep quality with some ethnic nuances [86-89]. Overall evidence shows that the efficacy of phytoestrogen intervention for treating HF and insomnia is not conclusive or generalizable.
Pharmacological non-hormonal interventions
It is widely accepted that serotonin and norepinephrine are implicated in the generation of HF. Therefore, antidepressants are an alternative for those women searching non-hormonal treatments. Evidence shows that women treated with selective serotonin re-uptake inhibitors (SSRI) (e.g., fluoxetine and paroxetine), and selective norepinephrine re-uptake inhibitors (SNRI) (e.g., venlafaxine) decrease the frequency and severity of their HF [90]. However, these treatments are not recommended for patients with breast cancer. It has been reported that SSRI and SNRI reduce tamoxifen efficacy by inhibiting the CYP2D6, preventing the bioactivation of tamoxifen to its active metabolite [91,92]. The effect of SSRI and SNRI on sleep come from their inhibitory effect on both serotonin and norepinephrine reuptake [93]. Specifically, and during the first week of treatment, some classes of SSRI may deteriorate sleep quality leading to insomnia or somnolence complaints, and then daytime dysfunction whereas others can promote sleep [94,95]. In addition, SSRI and SNRI present other side effects such as nausea, dizziness, headache, and dry mouth. Moreover, SSRI and SNRI based treatments are contraindicated in neuroleptic patients, those with the serotonergic syndrome or those using monoamine oxidase (MAO) inhibitors. The moderate response and side effects associated with SSRI and SNRI based treatments frequently cause patient’s non-compliance [96].
In addition, antihypertensive drugs such as clonidine (alpha-adrenergic agonist) could be considered a non-hormonal alternative for VMS and sleep disorders. However, the efficacy of clonidine treatment for HF frequency is modest [97,98]. The possible mechanism of action could involve the reduction of norepinephrine levels, which are elevated as consequence of estrogen withdrawal and HF [99]. Despite this, clonidine treatment leads to the appearance of side effects including dizziness, light-headedness, hypotension, and dry mouth, which make it a less desirable alternative [99] and is not currently recommended [100]. Concerning sleep disorders most of the studies have been performed in children and adolescents with attention-deficit hyperactivity disorder (ADHD). Miyazaki et al. [101] showed that low doses of clonidine increased rapid eye movement (REM) sleep. However, now it is known that clonidine alters NREM/REM sleep cycle by reducing REM sleep in a dose dependent manner as it reduces the release of norepinephrine thus maintaining the activity of REM on neurons [101]. Further investigation is needed regarding the efficacy of clonidine for the treatment of sleep disorders during menopausal stages [102,103].
Other proven pharmacological non-hormonal treatments for HF and sleep disorders are anti-epileptic drugs such as gabapentin. This molecule is an analogue of the gamma-aminobutyric acid (GABA) used as an anticonvulsant. This option reduces the frequency of HF by 47-71% in four clinical trials [104-106]. Moreover, GABA based treatment has shown effectivity in either menopausal or in tamoxifen-induced menopausal women [107] and could be considered as a treatment option for HF in women with breast cancer [108-110]. Nevertheless, GABA’s associated side effects include somnolence, ataxia, and dizziness. Evidence from a meta-analysis, which compared single-agent gabapentin with placebo for treating HF, shows that adverse events were significantly more frequent among those taking gabapentin than among those taking the placebo [111]. Concerning sleep disorders, it has been demonstrated that gabapentin enhances slow-wave sleep in patients with primary insomnia, improves sleep quality by elevating sleep efficiency and decreasing spontaneous arousal [112]. Similarly, patients on single doses of gabapentin between 250 and 500 mg showed significantly longer sleep duration and greater depth than patients on placebo [113].
Finally, the effects of benzodiazepines on HF frequency and severity have not been studied. However, treatments based on short half-life benzodiazepines are the main option for sleep disorders including chronic insomnia and sleep-onset insomnia. Secondary side effects after benzodiazepine discontinuation include rebound insomnia and withdrawal symptoms [114]. Other molecules such as selective dual orexin receptor antagonist (suvorexant) or melatonin agonists (Ramelteon) are only efficient for insomnia.
Even though there are several options for either sleep disorders or VMS not too many represent an integral option for treating VMS and sleep disorders simultaneously. In conclusion, pharmacological options present side effects outweighing their benefits. Therefore, it is necessary to evaluate non-pharmacological alternatives in order to avoid the side effects and potential toxicity of pharmacological treatments. These alternatives could improve patients’ long-term compliance when treating simultaneously HF and sleep disturbances.
Non-pharmacological treatments for VMS and sleep disorders
Lifestyle changes, behavioral therapies, holistic techniques, food supplements, and herbal therapies are considered as non-pharmacological interventions. In general, lifestyle interventions include habits that promote reductions in core body temperature. For example, these treatments advocate reductions and avoidance of habits that involve alcohol, and hot and spicy food. However, there are no clinical studies evidencing any significant effect of adopting these habits on HF severity and frequency [115].
Concerning sleep disorders, European guidelines propose good hygiene rules: physical exercise, controlling substance use and avoiding environmental factors that disrupt sleep, such as light, temperature, and noise [116]. Moreover, practicing moderate exercise can contribute to decrease VMS during the menopause improving quality of life, cognitive and physical function, and reducing all mortality causes [117]. Evidence from longitudinal and cross-sectional studies shows that peri- and postmenopausal women who work out on a daily basis exhibit lower HF frequency than sedentary ones, independently of their ethnicity [13,118-122]. Similarly, evidence shows that sedentary habits tend to increase levels of cholesterol, triglycerides, and apolipoprotein A, which lead to an increase of HF frequency and severity [123]. Likewise, in obese women, weight loss might be associated with a decrease or the total elimination of HF [124]. Consistently, results from 4 randomized controlled trials have shown that weight loss may be effective in the management or resolution of sleep disorders including OSA (measured by changes in Apnea Hypoapnea Index) [125]. Likewise, physical activity also increases sleep efficiency and duration in mid-aged and elderly women, irrespective of the type of working-out practice and intensity [126]. For instance, yoga reduces vasomotor, psychological, somatic, and urogenital symptoms during both peri- and postmenopausal stages [127,128]. However, results on the effects of yoga on sleep disorders are still controversial [129,130]. Moreover, Wang et al. [131] reported that four randomized controlled trials revealed no evidence for the effects of yoga compared with the control group in improving the sleep quality (assessed with the PSQI) for peri/postmenopausal women using. Analogously, holistic breathing techniques including paced respiration, hypnosis and acupuncture show different results regarding the frequency and severity of HF and their associated sleep disorders [132]. Accordingly, various studies show how slow-paced respiration or hypnosis significantly reduces frequency and intensity of HF in menopausal women [133-135]. Finally, acupuncture could reduce the frequency and severity of HF and improve menopausal women’s quality of life [136]. Likewise, this treatment also improves sleep quality in peri- and postmenopausal women [137].
Complementary medicine as Cognitive Behavioral Therapy (CBT) has a positive effect on both HF and sleep disorders and consequently on depression in postmenopausal and breast cancer survivors [138,139]. This intervention implies behavioral activation and changing negative thoughts. Mindfulness-Based Cognitive Therapy (MBCT) not only reduces symptoms of depression, stress, and anxiety, but also HF during the menopausal transition, in both healthy menopausal and those with breast cancer [139-142]. Then CBT shows efficacy in general population reducing both hot flushes and insomnia. Likewise, CBT is currently recommended as a first line treatment in healthy midlife women with insomnia and moderate VMS [141].
Herbal therapies have been studied as a natural alternative to treat HF and sleep disorders. However, in some cases the effectiveness and their mode of action is not fully demonstrated. Particularly, the active ingredients of black cohosh (Actaea racemosae) could act as selective estrogen receptor agonists, dopaminergic and serotonergic pathway modulators, as well as acting as antioxidant and anti-inflammatory agents [143]. Accordingly, a Cochrane review based on 16 randomized clinical trials in 2,027 perimenopausal or postmenopausal women, report that there was no significant difference in HF frequency between women treated with black cohosh and those with placebo [144]. It seems that the effectiveness of black cohosh depends on the type of used extract. The hydroalcoholic extract BNO 1055 has been proposed as a therapy for HF and sweating. Jarry et al. [145] reported lack of binding activity to ER-α or ER-β receptors with this extract. Moreover, BNO 1055 extract is also well suited to improve sleeping quality via the activation of the GABAergic system. However, potential side effects could occur such as gastrointestinal disorders (e.g., upper abdominal symptoms, diarrhea), allergic skin reactions (e.g., hives, itching, skin rash), facial or peripheral swelling (facial or peripheral edema), and weight gain. Regarding side effects, suspected hepatotoxicity was previously reported, but a meta-analysis of five randomized, double-blind, controlled clinical trials addressing 1,020 women showed no evidence that black cohosh has any adverse effect on the liver function [146].
Another available natural product is the Purified and Specific Cytoplasmic Pollen Extract (known as PureCyTonin®) which has demonstrated in several trials its efficacy at reducing HF, night sweats, and sleep disorders in perimenopausal and menopausal women [147-152]. Moreover, evidence from several in vitro and in vivo tests show that these extracts do not have either estrogenic action or uterotrophic effects and does not interfere with tamoxifen efficacy [153] which has led to propose it as a safe non-hormonal alternative treatment to MHT for menopausal symptoms [154].
The reduction in symptom scores reported from postmenopausal women treated with Purified and Specific Cytoplasmic Pollen Extract is not mediated by estrogen or estrogen-like pathways as shown in the study of Seeger et al. [155] in which no proliferation of MCF-7 cells could be demonstrated. Therefore, the Purified and Specific Cytoplasmic Pollen Extract could be a non-hormonal alternative for managing menopausal symptoms in cancer survivors and for women with contraindications to MHT [156,157]. Concerning sleep disturbances, patients treated with Purified and Specific Cytoplasmic Pollen Extract showed significant decrease of 48.5% in the frequency of HF and 50.1% in sleep disturbances and mood disorders [158]. Similar results have been reported by Lello et al. [159] who evaluated the effect of pollen extracts in 108 menopausal women. Their results show that HF, night sweats, difficulties in falling asleep, and fatigue were significantly reduced without presenting side effects [159].
Recently, a comparison between the effect of pollen extracts and isoflavones in sleep disorders in menopausal women showed significant greater improvement in sleep quality for the pollen treated group compared to the isoflavone group at both three (-24.7% versus -9.3%, p<0.001) and six months (-52.9% vs -4.0%; p<0.001), mainly for the scores related to subjective sleep quality, sleep latency and habitual sleep efficiency. Pollen extracts achieve a greater improvement of HF, sleep disturbances, and menopause-related symptoms than soy isoflavones and it is mainly effective when the quality of sleep is the most disturbing complaint [160]. Concerning the mode of action of Purified and Specific Cytoplasmic Pollen Extract, it seems to act on the hypothalamic thermoregulation center where HF are generated, acting similar to SSRI yet without their side effects [161]. Additionally, tryptophan, a precursor of serotonin, is present in the extract amount. The average measured level of tryptophan in pollen extracts is about 0.09 mg/tablet [162]. Thus, pollen extract appears to maintain the availability of serotonin in hypothalamic serotonergic neurons, partially explaining its efficacy in HF and sleep disturbances.
There are other non-pharmacological /non-hormonal alternative options such as nutraceuticals. Reported evidence comes from the effects of L-tryptophan, melatonin, and magnesium on HF and sleep disorders. However, in patients with breast, or cervical cancer who develop VSM, asthenia and insomnia, nutritional tryptophan supplementation at a dosage of 3 g per day is well tolerated, improving these symptoms and thus quality of life [163]. The effects of 5-Hydroxytriptophan (5-HTP), immediate precursor of serotonin, have been studied in trials with a dosage of 150 mg/day showing no significant amelioration of the frequency of HF [164]. Concerning sleep, scientific evidence concludes that tryptophan reduces sleep latency and increases subjective rating of sleepiness during the day. Tryptophan mechanism of action can be through melatonin enhancement of serotonin effects [165].
Melatonin based nutraceutical treatments for sleep disorders have been tested in adults and children. Melatonin interventions show little dependence and habituation without aftereffects and general minor side effects. Different meta-analyses show that doses of melatonin between 3 to 6 mg significantly improve sleep compared to placebo, reducing sleep onset latency, increasing total sleep time, and improving overall sleep quality in patients with primary sleep disorders [166]. Furthermore, melatonin formulations in doses from 1 mg to 1.9 mg have proven efficacy and they are accepted in some European countries [167].
Moreover, a randomized placebo-controlled study among breast cancer survivors demonstrated that melatonin was associated with an improvement in subjective reported sleep quality, without any significant adverse effects [168].
In a general trend, woman with sleep problems improve their sleep quality with small doses of melatonin [169]. However, evidence shows a non-role of melatonin in reducing VMS [170].
Finally, magnesium is likely to be a reasonable causal link between VMS and the menopause. However, magnesium supplementation has been tested in menopausal women who have had breast cancer failing to reduce HF [171]. On the other hand, Abbasi et al. [172] have shown how low magnesium levels are associated with nervous excitability and poor sleep quality. Particularly these authors examined the independent role of magnesium in the treatment of insomnia. Supplementation of magnesium appears to improve subjective measures of insomnia such as Insomnia Severity Index Score, sleep efficiency, sleep time and sleep onset latency, early morning awakening.
Conclusions
This review reports how the transition to menopause and post menopause involve a wide spectrum of endocrine, health and age associated changes interacting simultaneously, which affect women’s sleep quality. Several lines of evidence show reductions in HF frequency and severity in women treated with MHT, improving their sleep quality. Even though there are several MHT alternatives options for either sleep disorders or VMS not many represent an option for treating both symptoms simultaneously. Nevertheless, pharmacological options present side effects outweighing their benefits. Non-pharmacological treatments, such as hygienic dietary measures, behavioral therapies, holistic technics, food supplements, and herbal therapies are considered as natural options. Particularly, CBT and purified and specific cytoplasmic pollen extracts could cover both HF and sleep disorders without side effects. However, it is necessary to continue evaluating non-pharmacological alternatives in terms of their efficacy and safety for the treatment of menopausal HF and sleep disorders.