Introduction
Abnormal uterine bleeding (AUB) is defined as irregularities in the menstrual cycle involving menstrual volume, duration, frequency and regularity in a non-pregnant woman. It is the most common gynaecologic complaint of adolescents admitted to a hospital, affecting up to 20% of reproductive-aged females, with a higher incidence during the adolescence [1]. AUB is an important female adolescent health care problem, causing serious implications for quality of life in young girls, as it significantly affects school attendance and social activity participation [2].
In adolescents, during the first 2-3 postmenarcheal years, menstrual cycle normally should occur every 21-45 days, menses lasting for 2-7 days with an average blood loss of 30-40 mL, requiring the use of 3-6 pads or tampons per day or 10-15 soaked pads or tampons per cycle [3].
The International Federation of Gynaecology and Obstetrics (FIGO) provides a PALM-COEIN classification system for causes of the AUB in non-gravid women [4]. The most common cause of the pathology is ovulatory dysfunction because of the immaturity of the hypothalamic-pituitary-ovarian (HPO) axis. Another leading etiology is various coagulopathies, including von Willebrand disease (vWD), other coagulation factor deficiencies, immune thrombocytopenia, platelet dysfunction and others. Less frequent causes such as adenomyosis, pregnancy, sexual trauma and sexually transmitted infections (STI) should also be taken into account in the diagnostic process. Structural causes of the AUB are extremely rare in the adolescent population.
Heavy menstrual bleeding (HMB) is the most frequent symptom of AUB, with a prevalence rate as high as 30% among adolescents presenting to the gynaecologist [5]. HMB refers to bleeding lasting more than 7 days and/or a blood loss of greater than 80 mL per menstrual cycle, causing symptomatic anemia [6].
Ovulatory dysfunction
For ovulation to occur regularly the normal functioning of the HPO axis is necessary. Normally, gonadotropin releasing hormone (GnRH) is synthesized and released by hypothalamus in a pulsatile fashion. GnRH secretion is driven by kisspeptin from kisspeptin-neurokinin B-dynorphin (KNDy) neurons of the hypothalamus. Kisspeptin release is possibly triggered or facilitated by neurokinin B (NKB) signaling.
When GnRH is released, it stimulates follicle stimulating hormone (FSH) and luteinizing hormone (LH) secretion from the hypophysis. The rise in LH concentrations stimulates the thecal cells in the ovaries to produce androgens while FSH stimulates the granulosa cells to convert androgens to estradiol (E2). During the follicular phase, E2 level increases and when it reaches the critical concentration (>200 pg/mL for two days) GnRH rises with positive feedback and LH surge occurs activating proteolytic hormones, which cause the follicular rupture and luteinisation of the granulosa and theca cells. This leads to a significant increase in progesterone production. E2 causes endometrial epithelial cell proliferation, gland growth and vascularization while increasing progesterone stabilizes the endometrium. The drop in estradiol and progesterone after the demise of the corpus luteum leads to endometrial necrosis and bleeding.
In up to 95% of adolescents with AUB anovulatory cycles are due to an immature HPO axis [7], although the pathophysiology is not well established. During the first years after menarche, menstrual cycles have longer follicular phases, fail to form a preovulatory follicle to secrete sufficient levels of E2 to elicit a proper LH surge. Furthermore, the positive feedback of E2 to LH is not functioning properly because of the insufficient pituitary potential. This results in absence of ovulation and an aluteal or inadequate luteal phase. In the absence of the corpus luteum only estrogen and no progesterone is produced. Unopposed estrogens induce endometrial proliferation, absence of progesterone leads to lack of endometrial stability which results in abnormal shedding of the endometrium in both timing and amount [8].
Moreover, ovulatory dysfunction among adolescents can be caused by polycystic ovary syndrome (PCOS). According to Benjamins [9], 30% of PCOS patients experience abnormal uterine bleeding.
Coagulopathies
An underlying coagulopathy is the second most common cause of AUB among teenagers. As stated by various authors from 22 to 50% of adolescents presenting with HMB are diagnosed with an underlying disorder of the haemostasis [10-13]. vWD and platelet dysfunction are the most frequent conditions.
vWD is an autosomally inherited congenital bleeding disorder, presenting with deficiency of the von Willebrand factor, which is a protein critical for platelet adhesion and protection against coagulant factor degradation. Although the prevalence of vWD in the general population is low (1%) [13], it is a quite common disorder among adolescents with AUB, with a frequency ranging between 3 and 36% in this population [13,14]. Despite HMB experienced by 74-92% of women with vWD [15], the disorder may also present with epistaxis, bruising, gingival, gastrointestinal and postoperative bleeding or bleeding after dental extraction. Therefore, it is important to ask about the presence of these symptoms in the medical history.
Another haemostatic abnormality that seems to be even more common among teenagers is platelet disorders, with a prevalence ranging between 3 and 44% in AUB patients [13]. Platelet disorders include disorders of platelet number and disorders of platelet function, mostly failure of proper adhesion, which can be inherited or acquired. Clinical features of the disorder are indistinguishable from those found in individuals with vWD or other coagulopathies.
Other etiological factors
Despite the fact that anovulatory cycles and haemostatic abnormalities are the most common causes of AUB, other possible etiological factors should be taken into consideration when making a differential diagnosis.
First of all, abnormal bleeding from the uterine corpus can be caused by pregnancy and pregnancy-related complications. That is important, because around 50% of female teenagers are sexually active and only 78% of them are using contraception. Furthermore, STIs are also a growing concern for adolescents. Pathogens, responsible for STI, cause inflammation of the genital tract that leads to intermenstrual or excessive menstrual bleeding.
Endocrinopathies, such as PCOS, thyroid disease, adrenal problems and hyperprolactinemia can also cause AUB. For instance, HMB may be present in 50-80% of individuals with hypothyroid conditions [16].
Rarely, AUB in adolescents may be associated with sexual abuse, foreign bodies and genital tract structural disorders. It should be noted, however, that in these cases spotting is usually present rather than a HMB.
Other possible causes that are less frequent in the adolescent population can be the use of some medications, such as anticoagulants or hormonal drugs.
Clinical evaluation
Clinical evaluation should be performed through medical history, physical examination, laboratory testing and radiologic imaging.
Menstrual, sexual and medical history should be taken with the details of menarcheal age, menstrual regularity and duration, number of pads or tampons used per cycle. Particulars regarding patient’s systemic illnesses, past personal medical and family history and medication use are also important. Certainly, information regarding bleeding associated with surgeries and dental work, easy bruising and epistaxis, gum bleeding helps diagnosing underlying coagulopathies.
During a general physical examination, the assessment of Tanner staging and palpation of the abdomen should be performed. Also, it is important to check for signs of anemia, paying attention to bruising, petechiae, the presence of goiter and signs of androgen excess.
Assessment of the external genitalia, hymen and the lower part of the vagina should be considered – it is important to examine the vaginal opening in order to detect lesions, masses, septum or vaginal discharge.
For sexually active teenagers, pelvic examination with a speculum and transvaginal ultrasonography can be performed. According to Pecchioli et al. structural origin of the AUB is found in only 1.3% of females aged <18, suggesting that a pelvic ultrasound examination may not be even required in the initial investigation of AUB in the adolescent population [17].
Although structural causes of AUB are exceptionally rare in adolescents, pelvic ultrasound examination is informative, as it provides information regarding the uterine volume and endometrial thickness, which may help to choose the best treatment option [18]. As reported by Martire et al. [19] and Exacoustos et al. [20], in case of adenomyosis ultrasound signs can be found in 5% of adolescent women referred for sonographic examination highlighting the importance of this diagnostic method in cases of AUB. However, the value of the ultrasound examination mainly depends on the proficiency of the examiner. Transabdominal ultrasonography is a method of choice for evaluation of internal genital organs in adolescents prior to first intercourse.
Laboratory tests usually provide essential information of the underlying disorder and the severity of the bleeding. Pregnancy test, complete blood count with platelets, peripheral blood smear, ferritin, prothrombin time, activated partial thromboplastin time and fibrinogen should be performed. Patients with suspected coagulopathy should undergo testing for functional platelet deficiency or vWD. The panel for vWD should include plasma von Willebrand factor (vWF) antigen and functional tests for vWF and factor VIII activity [21]. If any sort of coagulopathy is suspected the patient should be referred to the haematologist.
For patients with suspected ovulatory disorder, it is important to perform hormonal tests, including thyroid stimulating hormone (TSH), FSH, LH and prolactin. However, required parameters should be decided on a case-by-case basis.
Furthermore, as the sexually adolescent population is at risk for STIs, evaluating for Neisseria gonorrhoeae and Chlamydia trachomatis is necessary.
Management
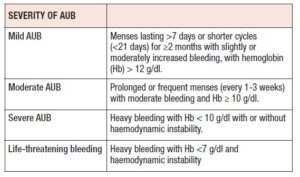
The principal goals for the management of an AUB are to stop the bleeding, to correct anaemia and to maintain normal menstrual cycles. The method of treatment to be chosen mainly depends on the severity of bleeding and anaemia. Classification of severity is given in the Table I.
Because anovulatory cycles are the most common aetiology of AUB, management recommendations should mainly focus on this.
Observation is usually enough for patients with mild AUB and normal hemoglobin (Hb) levels. It is important to inform the adolescent and the family regarding the condition and to propose possible treatment options. If bleeding is moderately increased and negatively affects the quality of life, non-steroidal anti-inflammatory drugs (NSAIDs) and tranexamic acid can be prescribed. Hormonal therapy can also be the treatment of choice, especially if the desire of contraception exists. Follow up in 3-6 months is recommended.
Management of moderate AUB depends on whether the bleeding is active or not. For adolescents currently not bleeding, progestin-only hormonal therapy is the method of choice as a maintenance regimen. Possible options are micronized progesterone (200 mg/day for the first 12 days of each calendar month if not allergic to peanuts), dydrogesterone (20 mg/day for the first 10 days of each calendar month), medroxyprogesterone acetate (10 mg/day for the first 10 days of each calendar month) or norethisterone acetate (2.5-5 mg/day for the first 5 to 10 days in every cycle). The latter is metabolized to ethinylestradiol and as a result is even more active on the endometrium. Monophasic oral contraceptives with a minimum of 30 mcg ethinylestradiol can be prescribed if there are no contraindications. Nonetheless, combined oral contraceptives (COCs) containing estradiol instead of ethinylestradiol (e.g., nomegestrol acetate/estradiol) can be effective in management of moderate AUB. However, there is not sufficient data on efficacy and safety of this therapy in the adolescent population. For patients who decline hormonal treatment, tranexamic acid can be recommended.
For actively bleeding adolescents with moderate AUB, COCs are the best choice. Monophasic COCs, containing minimum 30 mcg of ethinylestradiol are preferred, taking one pill every 8 hours until the bleeding stops (usually within 24-48 hours), then 1 pill every 12 hours for 2 days and then to continue with one pill per day for a total of at least 21 days. At the end of 21 days, seven days of placebo should be given or a pause performed.
Certainly, for patients with HMB resulting in anaemia supplemental iron and iron-rich foods should be recommended.
In case of severe AUB, all hemodynamically unstable patients with symptomatic anaemia and extremely low Hb levels (Hb <7 g/dL or <10 g/dL with active heavy bleeding) should be hospitalized. For initial management, monophasic oral contraceptives containing at least 30-35 mcg ethinylestradiol with a second-generation progesterone should be given with the regime of one pill every 6 hours until bleeding stops (usually within 24-48 hours). After, continue with one pill every 8 hour for 3 days, then one pill every 12 hours for 3 days and one pill per day continuously until Hb concentration is normal. When estrogens are contraindicated, progestin-only regimen can be used.
Surgical intervention is performed only in case of life-threatening bleeding.
Conflicts of Interest: The authors declare having no conflicts of interest.