Introduction
Depot medroxyprogesterone acetate (DMPA) is a contraceptive administered by intramuscular (dose of 150 mg/mL) or subcutaneous (dose of 104mg/0.65 mL) injection. These formulations are slowly released into the circulation from the injection site, thus DMPA is given every three months. It is known that medroxyprogesterone acetate inhibits the secretion of luteinizing hormone (LH) peak and, to a lesser extent, follicular-stimulating hormone (FSH) secretion but basal levels of these hormones remain similar to the luteal phase of a menstrual cycle. DMPA is indicated particularly for women with contraindications for estrogen therapy. Women treated with DMPA for several years have decreased serum estradiol concentrations [1]. This can contribute to the loss of bone mineral density (BMD), nevertheless there are some discrepancies in the data. There are also many analyses which assess the risk of venous thrombosis in patients using non oral contraceptives [2,3], although the most common side effects of DMPA administration are menstrual disturbances and weight gain [4].
Case description
The 39-year-old patient was admitted to the Department of Gynecological Endocrinology, Poznan University of Medical Sciences due to irregular menstrual cycles. The duration of the menstrual cycle fluctuated from 28 to 60 days and the menstruation length was 3 to 5 days. Patient denied heavy menstrual bleeding and painful menstruation. Menarche occurred at the age of 13 and since then until the DMPA treatment she had a regular menstrual cycle with a mean duration of 30 days. In the past she was treated with DMPA for about 10 years. The patient had the last dose of DMPA 2 years prior to the time of admission to the hospital. Restoration of menses appeared 1 year after the last dose. Previously she hadn’t used any other hormonal contraceptives.
It is worth mentioning that in her medical history we found the information that at the age of 37 she had suffered deep vein thrombosis in the lower extremities. It was followed by severe pain in the calves with accompanying swelling and redness. To make the diagnosis of thrombosis a doppler ultrasound was performed that revealed clots in the deep veins of the legs. The patient received treatment based on the guidelines of the National Society of Hematology. In the past, the patient underwent splenectomy due to the diagnosis of spherocytosis. Aside of the aforementioned she denied other surgeries or hospitalizations, and she didn’t take any long-term medications.
Her body mass index (BMI) was increased: 28.7 kg/m2 (height 1.73 m, weight 86 k). The patient did not report any weight changes in the previous six months. She performed moderate everyday activity without intensive training. Her diet was varied without any food intolerances or restrictions.
Hormonal tests
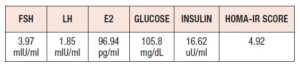
In Table I we presented hormonal results of the patient. By the time of hospital admission estradiol level was in normal range. We noticed impaired fasting glucose which was slightly elevated to 105.8 mg/dL and the insulin level was 16.62 µU/mL, thus, we diagnosed insulin resistance (IR). The homeostasis model assessment of insulin resistance (HOMA-IR score) value was 4.92 (cut-off point for IR diagnosis is 2.5).
As prolonged hypoestrogenism can lead to decreased bone density a dual energy X-ray absorptiometry was performed. Lumbar spine densitometry revealed osteopenia with a T-score at -1.0 and Z-score at -1.7. A T-score of –1 to –2.5 SD indicates osteopenia and a T-score of less than –2.5 SD indicates osteoporosis. The patient did not have a history of fragility fractures.
Transvaginal ultrasonography revealed a normal size uterus in anteflexion with a thin endometrium (5 mm) and the presence of a follicle of 24 mm in the right ovary. The appearance of the left ovary was normal.
Due to menstrual changes the patient was prescribed norethisterone acetate in the daily dose of 10 mg and due to IR, extended-release metformin was given in the dose of 500 mg daily to control serum glucose levels. We encouraged her to perform regular exercise, implement a low glycemic index diet and appropriate intake of calcium and vitamin D. Follow up appointments in our outpatient clinic were arranged.
Mini-review of the literature
DMPA is a very comfortable method of contraception, thus many women use it for a very long time. There is no evidence-based data regarding the limit of duration of such therapy, however, the U.S. Food and Drug Administration warns about loss of bone mineral density and considers that DMPA contraceptive injection should not be used for more than 2 years [5].
Menstruation changes
It is estimated that prolonged use of DMPA causes atrophy of the endometrium, which leads to amenorrhea in women using this form of contraception [1,6]. After 12 months of usage amenorrhea has been reported in 55% of women and after 1 month in 24-68% of women [7]. The serum concentration of DMPA may vary among women but a plateau of 1.0 ng/mL is achieved after about three months. After achieving the plateau, a gradual decline in concentration is observed, while ovulation may occur when DMPA concentrations decrease below 0.1 ng/mL. This is the presumed cause of the delayed return of fertility about 7 to 9 months after the last administration of DMPA [1,6]. Delayed return of menstrual cycles may be due to slower DMPA metabolism.
Loss of bone mineral density (BMD)
There are many conflicting data from various studies that assess the risk of BMD loss during DMPA use. The most common question in this regard is whether long-term treatment with DMPA is associated with BMD loss. The study conducted by Zeman et al. [8], that included 21 healthy women with a mean age of 31 years and a mean time of DMPA use of 7 years, revealed lower BMD when compared to baseline. BMD was measured by using Hologic dual-energy X-ray absorptiometry. The most significant loss was observed during the first 2 years of use and afterwards this decline gradually stabilized [8]. A population study from New Zealand which compared 30 current DMPA users (with minimal use of 5 years) with 30 premenopausal and 30 postmenopausal women showed significantly reduced BMD in the lumbar spine and in the femoral neck in women treated with DMPA. They concluded that estrogen deficiency induced by DMPA might have been correlated with BMD loss [9]. However, other longitudinal cross-sectional studies reveal that after discontinuing DMPA there is a large or even complete recovery of BMD. Scholes et al. [10] conducted a population-based prospective cohort study among 457 non-pregnant women, aged 18 to 39 years (183 DMPA users and 274 non-users). Every 6 months for 3 years BMD was measured by dual-energy x-ray absorptiometry. They found that BMD decreased among DMPA-users at the spine and total hip, but after discontinuation, a sizable increase in BMD was observed. Moreover, after 30 months there was no difference in mean BMD between both groups.
Taking this into consideration it is suspected that BMD loss after discontinuation of DMPA treatment is largely reversible. Another 7-year prospective, matched-cohort, clinical study compared BMD in DMPA users (n=248) with women using non hormonal contraception (n=360) for up to 240 weeks of treatment and 96 weeks after discontinuation. The results showed a decline in BMD during DMPA use and a significant recovery 96 weeks posttreatment [11]. In 2005, the World Health Organization convened a technical consultation regarding the effects of hormonal contraception on bone health. Experts concluded that there should be no restriction on the duration of DMPA use and that in women aged 18 to 45 the advantages of using this type of contraception generally outweigh the potential risk of fracture [12].
It is important to underline the role of fat tissue as a protective factor against bone density reduction, especially in postmenopausal women. Aromatization of androgens in the adipose tissue provides an extraovarian production of estrogens and constitutes its major source after the menopause [13]. Although the ovaries of women before menopause produce the majority of estrogens, the contribution of peripheral synthesis in the adipose tissue may be relevant, particularly among DMPA users. Studies have shown a positive correlation between BMD and both BMI and total fat mass [14]. Based on that we can assume that the amount of fat tissue might be a protective factor while using DMPA and overweight patients are at lower risk of BMD loss.
Observational studies have revealed that DMPA use can increase fracture risk [15,16]. Based on currently available data, the American College of Obstetricians and Gynecologists’ Committees on Adolescent Health Care and Gynecologic Practice state that concerns regarding the effects of DMPA on BMD and potential fracture risk should not prevent practitioners from prescribing DMPA or continuing use beyond 2 years [17].
Thromboembolism risk
Episodes of deep venous thrombosis among DMPA users are rare. Therefore, DMPA has not been advocated as the cause of thrombotic or thromboembolic disorders. van Hylckama Vlieg et al. [3] assessed the risk of venous thrombosis associated with the use of non-oral contraceptives (i.e., injectable DMPA, hormone levonorgestrel-releasing intrauterine devices (IUD), a contraceptive patch, or a contraceptive implant). They selected premenopausal women aged 18-50, 446 users and 1,146 controls and found that the risk of venous thrombosis was increased for injectable DMPA contraceptive users, while such a risk was not observed among levonorgestrel intrauterine device users.
On the other hand, the prospective study which included 39 healthy women aged 20-39 years, with a BMI < 30, who had never used DMPA, and who opted to use DMPA (21 women) or a copper IUD (18 women) showed that among new DMPA users D – dimer levels were lower and the time to peak thrombin generation was longer. Blood samples were obtained from all participants at baseline and at 6 and 12 months. According to the results, the authors suggest a positive profile against hypercoagulability [18].
It is known that DMPA can cause some unfavorable changes in the lipid profile when used for a long time, but there is some evidence that these changes level off during therapy. Berenson et al. [19] evaluated the effects of using DMPA or oral contraceptives (OCs) on serum lipid levels in 703 women (white, African-American, and Hispanic) in comparison to those using a non-hormonal birth control. Serum lipids were measured at baseline and every 6 months thereafter for 3 years. The patients who used DMPA were followed up for 2 additional years. They found that OC users experienced significantly greater increases in levels of triglycerides, total cholesterol, very low-density lipoprotein cholesterol (VLDL-C), and high-density lipoprotein cholesterol (HDL-C) when compared to non-hormonal-contraceptive users. Furthermore, among DMPA users, HDL-C levels initially decreased for 6 months but then returned to baseline. They revealed that after DMPA was discontinued, triglyceride, VLDL-C, and HDL-C levels were significantly higher in women who used OCs than in those who chose non-hormonal methods but these effects are temporary [19].
The American College of Obstetricians and Gynecologists, the Centers for Disease Control and Prevention Medical Eligibility Criteria for Contraceptive Use, and the World Health Organization Medical Eligibility Criteria for Contraceptive Use consider DMPA as an acceptable contraceptive option (Category 2) for women with known thrombogenic mutations or a history of deep venous thrombosis/pulmonary embolism, particularly those with uncomplicated events in whom the use of estrogen-progestin contraceptives is contraindicated [20]. However, package labeling for DMPA suggests that a prior history of venous thromboembolism should be considered as contraindication for DMPA use [21]. Additionally, having multiple cardiovascular risk factors or a history of stroke are contraindications for DMPA use.
Insulin and glucose levels
The risk of diabetes mellitus type 2 increases among women who use DMPA during breastfeeding, have increased baseline diabetes risk or triglyceride levels, or gain weight during its [21]. A prospective 12-month study from Brazil compared 31 DMPA users with 25 copper IUD users, matched for age and BMI [22]. BMI, waist circumference, fasting glucose, fasting insulin and HOMA-IR were evaluated at baseline and after 6 and 12 months of contraceptive use. They found that after twelve months, women who used DMPA had significantly higher BMI waist circumference values.
Berenson et al. [23] estimated the effect of using DMPA and OCs containing 20 micrograms of ethynilestradiol and 0.15 mg desogestrel on serum glucose and insulin levels. They measured fasting glucose and insulin levels on 703 women (white, African-American and Hispanic) at baseline and every 6 months thereafter for 3 years. Throughout 30 months there was a steady increase in serum glucose levels (2 mg/dL at 6 months to 3 mg/dL at 30 months) but then it leveled off. Serum insulin levels rose during the first 18 months of DMPA use (3 units at 6 months to 4 units at 18 months) and then remained constant. Furthermore, elevation of these parameters was more pronounced in obese and overweight users. They concluded that DMPA use but not OC use can lead to higher fasting glucose and insulin levels [23].
Discussion and conclusions
In the described case, we present a woman with adverse effects after long-term treatment with DMPA. There is a lack of accurate data regarding longitudinal follow-up of women treated with DMPA. Menstrual changes occur in all women using DMPA [24]. The longer duration of use, the higher possibility of amenorrhea [25]. We did not find any data answering the question of when menstruation returns after discontinuation of DMPA use. Discrepancy between the studies that assess BMD loss is probably due to the different measuring techniques (i.e., evaluated region) and/or small sample sizes.
Evidence supporting the association of long-term DMPA use and future BMD loss or risk of fractures is still controversial. Most of the available data have emphasized the reversibility of this phenomenon after discontinuing DMPA but there is a need to take into account additional risk factors such as smoking, low BMI, lack of performing exercise. Patients are recommended to perform regular exercise and intake calcium and vitamin D in order to aid BMD recovery.
Dianat et al. [26] performed a systematic review of publications regarding the side effects and health benefits of DMPA in order to provide adequate counseling. Their search yielded twenty-four studies (no randomized clinical trials) in order to answer two key questions: 1) What side effects are associated with progestin-only injectable contraceptive use? 2) What health benefits are associated with progestin-only injectable contraceptive use? Studies of moderate or high risk of bias suggest an association between DMPA use and weight gain, increased body fat mass, irregular bleeding, and amenorrhea. There was inconsistent or limited evidence for the association between DMPA use and mood or libido changes or decreased risk of cancers and tubal infertility. The author conclude that higher-quality research is needed to clarify DMPA's side effects and benefits. Hence, patients should be counseled taking into consideration the available evidence and recognizing the value of women's lived experiences.
Other long-acting contraceptives may share similar side effects as those observed with DMPA use. For instance, Pongsatha et al. [27] investigated the effects of long-term use of a subdermal single-rod contraceptive implant on BMD. They found that subdermal implant users had a significantly lower BMD at the distal radius and ulna in comparison to controls. Taking into consideration the negative influence of DMPA and implants on bone health, levonorgestrel-releasing intrauterine system (LNG-IUS) might be a preferable long-acting reversible contraceptive in some groups of patients. Indeed, LNG-IUS does not influence BMD negatively [28]. Abnormal menstrual bleeding has been related to implant and LNG-IUS use, representing the main reason for its early discontinuation [29].
In conclusion, until new consistent evidence regarding the potential adverse effects of DMPA is available, patients should be counseled in terms of the limitations related to long-term DMPA use and prescription be based in agreement to specific indications.
Conflict of interest statement: The authors declare having no conflicts of interest.