Introduction
Precocious puberty is commonly defined as the onset of secondary sexual characteristics in females before the age of 8 (2 to 2.5 standard deviations earlier than population norms) or as accelerated development before the age of 9 [1]. The incidence of precocious puberty in girls varies across populations. Central precocious puberty (CPP) is also known as gonadotropin-dependent precocious puberty and is caused by premature maturation of the hypothalamus-pituitary-gonadal axis. A 2000 study reported that the prevalence of idiopathic central precocious puberty (ICPP) is 80-90% of cases in females [1,2]. Tanner staging and the assessment of skeletal maturation are fundamental tools for the diagnosis of precocious puberty. Baseline gonadotropin profile is rarely diagnostic even if more sensitive luteinizing hormone (LH) assays are under study [3,4]. Instead, gonadotropin testing before and after Gonadotropin-releasing hormone (GnRH) administration is often performed for definitive diagnosis (GnRH test) [2,3].
According to the 2008 consensus statement of the American Academy of Pediatrics, LH levels provide more information than follicle stimulating hormone (FSH) levels. However, the LH/FSH ratio after stimulation can help distinguish progressive ICPP (which usually has higher LH/FSH ratios) from non-progressive variants that do not require therapy [2,4]. Moreover, prepubertal blood levels of estradiol (E2) are often very low, even below 1 pg/mL. They can only be detected with more sensitive techniques such as chromatography and tandem mass spectrometry [2]. Since E2 is usually undetectable with the commonly available assays in prepubertal patients, the presence of measurable blood E2 may add information to support the diagnosis of precocious puberty but is not predictive [2,5-7].
Endogenous estrogens can be metabolized in the liver via several pathways which mainly involve hydroxylation pathways. Estrogens are excreted in the urine both in a hydroxylated form and as estradiol, estrone, and estriol in different concentrations [8,9]. E2 is also excreted in the urine in the form of estrone glucuronides, 2-hydroxyestrone, estradiol (5.2-7.5%), estriol, and 16alpha-hydroxyestrone [6,7].
Taking into account that children are reluctant to blood withdrawals, urinary hormone tests should be considered. According to the literature, urinary E2 values are correlated with those found in blood, although the exact percentage they represent is unclear [10,11].
The role of urinary gonadotropins in the evaluation of patients with ICPP has been studied for years. Many studies, even recently, have shown how urinary LH measurement is useful for diagnosing ICPP [12-19]. Sexual maturation is accompanied by increased LH and FSH urinary excretion. According to the literature, LH concentrations in early morning urine samples may reflect nocturnal pulsatile secretion which is markedly increased in both amplitude and frequency during pubertal development [20]. Thus, it is clear how urinary gonadotropins may play a role as one of the potential alternative approaches in the diagnosis and follow-up of ICPP patients receiving GnRH analog therapy [10,17,18,21].
Conversely, the use of the urine E2 test was not often considered in favor of the blood test. In particular, based on a study conducted by Norjavaara et al. [22], we can hypothesize that the morning E2 urinary assay may reflect nocturnal gonadotropin peak in girls during pubertal development. The study concluded that the nocturnal increase in gonadotropin secretion during puberty is accompanied by an increase in morning serum E2 secretion [22]. This could also be reflected in urinary E2 dosages.
Correlation between E2 urinary values before and after GnRH analog therapy could indicate a relationship with estrogen production expressed as endometrial thickness and vulvar estrogenization.
The aim of the present study was to evaluate the usefulness of urinary E2 assay as a tool for the diagnosis and management of precocious puberty in young girls.
This minimally invasive and easily applicable method could be inserted, as a routine tool to evaluate patients with suspected precocious puberty.
Material and methods
A retrospective review of outpatient records was performed regarding female patients admitted for suspected precocious puberty between January 2017 and June 2021. A total of 153 medical records were analyzed by three of the main authors (A.T, C.T, and F.P). Patients aged between 4 years and six months to 9 years were selected. Inclusion criteria were the suspicion of precocious puberty and the age of less than 9 years. Exclusion criteria were: peripheral precocious puberty, organic causes of precocious puberty and GnRH analog therapy at the time of the first visit. Patients who did not meet the criteria were excluded. Final evaluations were performed in 111 patients. Data collected anonymously were reviewed for age at diagnosis, blood assay, radiological images (such as radiographs of the wrist and transabdominal ultrasound of the internal genitalia) and urinary estradiol before and after GnRH analog therapy.
The urinary E2 values were obtained from the mean of the values of 3 urinary estradiol samples. The urine sample was collected as a first-morning void into a lidded container for 3 consecutive days. We collected urine samples every 3-6 months of follow-up. The samples were stored by freezing until they were processed in 2 stages: on the 1st day, they were subjected to hydrolysis, while on the 2nd day an extraction was carried out with subsequent dosage with the immunoassay method with Electro-Chemiluminescence Immunoassay (ECLIA). The final value was calculated as the ratio of E2 to creatinine urinary, and expressed in nmoles E2/g creatinine.
Serum E2 assay was performed with the same methodology as the urinary test and expressed in pg/mL. Ultrasound scans were performed using a transabdominal probe in a full-bladder state by two experienced sonographers using a Toshiba Aplio 400 device (Toshiba Medical Systems Corporation, Otawara, Japan), equipped with a 3.5 mHz convex transducer and a 12 MHz linear probe a convex transducer.
Measurements of the ovaries were made in longitudinal, transverse, and anterior-posterior views. The same was done for the measurement of the uterus. In cases where the ovarian parenchyma showed follicles their presence was recorded. Ovarian and uterine volume were calculated by an ultrasound program. Triptorelin (Decapeptyl®, Ferring) has been used for the treatment of precocious puberty. The decision to treat was made after evaluation of bone maturation, physical examination, maturation of the internal genitalia, and GnRH test if required.
The GnRH test was performed by administering 0.1 mg/m2 of triptorelin subcutaneously. Sampling of blood LH and FSH values were performed 60 and 120 minutes after administration of the GnRH analog. The test is considered positive if the LH values at the peak are greater than 5 IU/L. For LH values between 3.3 and 5 IU/L the test is considered suspicious. The test is considered positive even if the LH/FSH ratio at the peak is greater than 0.66. Patient data were collected and encrypted in an Excel® database (Office 365).
Statistical analysis
Data analysis was performed using SPSS statistical software, version 26 (IBM statistics). Data with normal distribution were expressed as mean ± standard deviation (SD), and for comparisons the t-test for paired samples was used (Student's t-test). Count data were expressed by n (%), and the Chi-square test was used for comparisons. For all analyses, a p value of < 0.05 was considered significant.
The threshold level for urinary E2 concentration to be used as a candidate marker for precocious puberty was obtained using the R package thresholdROC [23]. Patients were classified as positive and negative and the corresponding urine estradiol concentration was used to configure the thres2 function, assuming a disease prevalence (rho) of 1: 5000 [4], a significance level of 0.05 and the "unequal" method due to an unequal variance in samples as determined by the F-test (p < 0.001).
Results
Data from 111 patients (of whom 35 were treated) with precocious puberty were analyzed. Evaluated patients had a mean age of 7.8 ± 1.04 years, average birthweight of 3174 ± 472 g, and the average birthweight percentile was 29th according to growth curves [24].
Forty-one patients performed the GnRH test, 37 of whom were prescribed therapy with GnRh analog. The parents of two of these patients did not provide consent to be treated. Therefore, thirty-five patients started triptorelin therapy for a mean of 19 months (19 ± 11 months, range 6-40 months). Among the patients who performed the GnRH test, four had no indications for treatment and continued follow-up.
The treated group had a mean age at first visit of 7.7 years. The average birthweight was 3145 ± 544 g, and average birthweight percentile was 29th according to growth curves [24].
Among patients diagnosed with ICPP, 8 showed vulvar estrogenization (22.9%). In 5 out of 35 patients, axillary hair was found (14.3%). The mean height of the patients who received therapy was 129.7 cm, approximately at the 69th percentile according to growth curves [11]. Mean weight of treated patients was 29.5 kg (73rd percentile) [24].
Mean ovarian volume (first ultrasound examination) of the whole study population (n=111) was 2.6 cm3 while the mean uterine volume was 2.4 cm3. Mean ovarian volume of treated patients at the first ultrasound examination was 3.3 cm3, while the mean uterine volume was 3.0 cm3. Upon ultrasound examination 92 of 111 patients showed the presence of follicles within the ovarian parenchyma. All patients diagnosed with ICPP had ovaries with follicles and approximately 50% (n = 17) had an ovarian follicle larger than 7 mm.
None of the patients undergoing GnRH analog therapy experienced menarche before discontinuation of therapy. Baseline urinary E2 values were available in 71 out of 111 patients, while, among 25 out 35 patients undergoing treatment E2 urinary values were available. Serum E2 values at diagnosis were available in 32 patients.
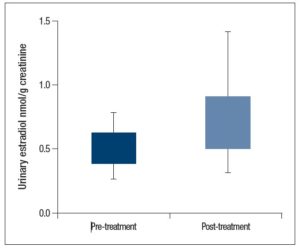
Mean pre-treatment urinary E2 values were 0.63 ± 0.34 nmol/g creatinine, while the mean post-treatment E2 values were 0.51 ± 0.30 nmol/g creatinine. We found a significant reduction in post-treatment urinary E2 (p = 0.027) as shown in Figure 1. We also compared serum E2 with urinary E2, this analysis showed a direct association between serum and urinary E2 values (p = 0.002).
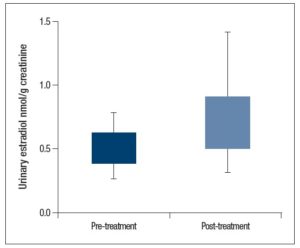
Further analysis showed that there was a significant difference (p = 0.013) when comparing mean baseline urinary E2 levels between the patients in which treatment was indicated (0.68 ± 0.40 nmol/g creatinine) and those in which it was not indicated (0.46 ± 0.24 nmol/g creatinine; Figure 2).
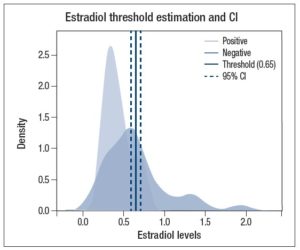
Using threshold ROC program, we obtained an estimated threshold of 0.65 nmol/g creatinine with a confidence interval (delta method) of 0.59-0.71, as shown in Figure 3.
In this case, the urinary E2 value ≥ 0.78 had a sensitivity of 100% and a specificity of 63%, to identify patients diagnosed with precocious puberty and therefore candidates for treatment.
When considering the total population of patients (n = 111), there was also a significant correlation between mean basal urinary E2 levels and endometrial thickness (p= 0.018). On the other hand, there was no significant correlation between the mean basal urinary E2 levels and uterine length (p= 0.423) or mean ovarian volume (p= 0.129).
Discussion
The goal of therapy in children with ICPP is the suppression of gonadal secretion of sex steroids to decrease bone maturation and, consequently, improve final height making it more similar to the target height. Monitoring the suppressive effect of therapy requires the evaluation of multiple complementary parameters. Clinical investigations, growth, and physical examination, are readily available, but lack the necessary sensitivity and specificity [25].
Pelvic ultrasound is a sensitive and specific supplementary method of monitoring girls with ICPP treated with GnRH analog, but the value of the investigation depends on the experience of the operator and the quality of the equipment. Some patients have abdominal adipose tissue which can make ultrasound examination difficult. Furthermore, in these patients, the examination is performed transabdominally, with a full bladder, and sometimes the patients may experience discomfort.
Serum assays of pituitary gonadotropins and gonadal sex steroids are troublesome, most of the plasma assay methods are not sensitive enough to distinguish between truly prepubescent values and those indicative of early pubertal development. Indeed, for the E2 assay, only the most sensitive techniques (tandem mass spectrometry) can detect prepubertal levels which can be 1 pg/mL (3.7 pmol/L) [2]. E2 in prepubertal patients is usually undetectable with commonly available dosaging methods. Therefore, measurable E2 usually only confirms early puberty. For this reason, the measurement of sex hormones can add information to support the diagnosis but is not conclusive [26,27]. Similarly, direct plasma E2 measurements are generally not adequate to confirm that ovarian steroid secretion has been reliably suppressed during follow-up [2].
The follow-up of patients treated with GnRH analog includes various clinical, instrumental, and laboratory methods, some of which are not very specific or operator dependent.
In this study, we evaluated the role of urinary E2 in the follow-up of GnRH analog treated ICPP patients and its possible use in the diagnosis of ICPP.
Our data shows that there is a significant difference between urinary E2 values before and after therapy. Indeed, post-treatment urinary values are lower, demonstrating that the GnRH analog therapy was effective.
Furthermore, we compared the urinary E2 values of the patients who were indicated for treatment according to other parameters (GnRH test, bone age, pelvic ultrasound), with those who were not. Again, our data showed a significant difference, particularly patients in whom treatment was indicated higher urinary estradiol values were found.
Our data also showed a significant relationship between progressive endometrial thickness and urinary E2 levels. This result emphasizes the relationship between serum E2 and urinary E2 (also confirmed by our data). This finding may further confirm how urinary E2 can be a useful tool for the evaluation of patients with ICPP. Since, urinary E2 levels are closely related to serum E2 values, it does not involve stressful tests for pediatric patients. Conversely, there was no relationship between urinary E2 values and the volume of the internal genitalia (ovaries and uterus). We could assume that the effect of E2 on the endometrium is prompter than the effect on the uterus that could result from cumulative estrogen secretion. Furthermore, contrary to what emerges from other studies reported in the literature, there is no relationship between birth weight, or the percentile of birth weight and ovarian or uterine volume [28,29].
Finally, we obtained a threshold ROC which helped us determine a cut-off value for urinary E2 according to our data. This value needs further study but may provide a tool to focus healthcare professionals on those patients who are at most risk.
We would like to emphasize that this method is minimally invasive for patients, furthermore, urinary E2 is an easy to perform analysis with a low implementation cost. In particular, the urinary E2 assay has a similar cost to the blood estradiol assay and it is about 5 times lower than the GnRH test.
Due to frequent injections and possible side effects, parents of patients are often reluctant to start therapy with GnRH analog. Urinary E2 is an additional tool that sometimes allows for greater compliance of patient's parents. Indeed, the relationship between serum and urinary E2 values demonstrates that urinary measurement can be equally reliable for the clinician and less discomforting for patients.
To our knowledge, urinary E2 has been described for the diagnosis and follow-up of patients with precocious puberty in only a few studies [30]. In particular, in this article by Tanaka et al. [30], 24-hour urine collection was used effectively. Our data were obtained from the average of three samples collected in the morning of 3 different consecutive days. This study is one of the very few that evaluates urinary E2 as a tool for the follow-up of patients with ICPP.
We focused on the usefulness and pitfalls of evaluating the efficacy of urinary E2 as a tool in patients with ICPP. In our opinion, this simple used tool could suggest the level of estrogenization of both internal and external genitalia and help in the diagnosis and follow up of patients with ICPP. The urinary E2 assay could also be used as a tool to assess the degree of treatment success. This minimally invasive and easy-to-apply method could be inserted, after further studies, as a routine tool for the diagnosis and follow-up of patients with suspected precocious puberty.
Acknowledgments: None.
Conflict of Interest Statement: All authors declare having no financial, personal or professional competing interests.