Introduction
In women, there are three circulating forms of estrogen with varying potencies. The most potent is estradiol, produced primarily by the ovaries, with a small percentage coming from the extra glandular conversion of testosterone to androstenedione. Estrone is mainly synthesized through peripheral conversion of androstenedione by the enzyme aromatase, while estriol, produced by the placenta, is the predominant estrogen in pregnancy.
In the fetus, high levels of estrogen are present due to conversion of fetal and maternal adrenal C19 steroids to estrogen by the placenta, and these levels drop steeply in the first few days of life. Estradiol levels are low in prepuberty and rise through puberty. Estrone levels rise early and reach a plateau by midpuberty. In the menstrual cycle estradiol levels peak at midcycle just before ovulation and then again at day 24, falling to baseline before menses (1).
The skin is highly sensitive to the effects of sex steroid hormones. Estrogen receptors are found throughout the skin, in sites including keratinocytes, sebaceous glands, hair follicles, dermal fibroblasts and melanocytes. The number of estrogen receptors is higher in women than in men. Estrogens have been shown to inhibit sebaceous activity but have little or no effect on apocrine glands. They increase dermal hyaluronic acid levels with a consequent increase in the water content of the dermis. They also reduce the breakdown of dermal collagen, probably by increasing the conversion of soluble to insoluble collagen. Estrogens also stimulate epidermal melanogenesis, contributing to the transient hyperpigmentation around the eyes and nipple that occurs premenstrually (2). Estrogens have also been shown to promote hair growth (3). In addition, they have anti-inflammatory properties and decrease the cutaneous response in delayed hypersensitivity reactions by increasing IFN-d mediated inhibition of Th-17 cells and suppressing TNF-a (4). The effects of estrogen on the skin have been evaluated mainly in studies on postmenopausal women.
Variations in estrogen levels throughout a woman’s lifespan, from birth through menstruation and pregnancy to menopause, can result in a spectrum of cutaneous manifestations.
Estrogen excess
The etiology of estrogen excess can either be either physiological or pathological.
Physiological
In females, elevated levels of estrogen occur with pregnancy. Pregnancy induces a number of changes in the skin, hair and nails which are regarded as physiological. It is important to recognize that these changes occur, so they are not confused with true skin diseases.
Cutaneous manifestations of estrogen excess in pregnancy
-
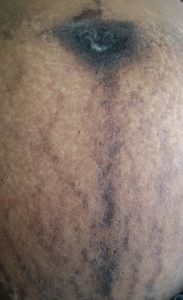
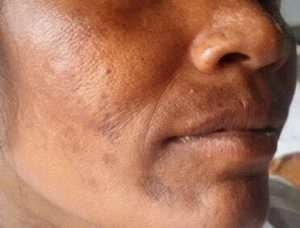
Pigmentation
The hormonal milieu of pregnancy promotes pigmentation – estrogen, progesterone and beta-endorphins promote melanocyte activity and corticotrophin-releasing hormone from the hypothalamus incites the placental production of melanocyte-stimulating hormone (5). Localized or generalized hyperpigmentation occurs in up to 90% of pregnant women. Hyperpigmentation of nipples, linea nigra (Figure 1), genitalia, preexisting naevi or malignant melanoma can occur in pregnancy. Melasma, which is characterized by the appearance of irregularly shaped hyperpigmented patches on the forehead, nose, upper lip, chin and neck (Figure 2), occurs in the second trimester in three quarters of pregnant women. It usually improves after delivery but can persist in 30% or recur with subsequent pregnancies. Persistent hyperpigmentation is more likely in patients with darker skin and frequent sun exposure.
-
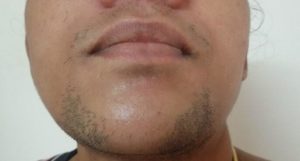
Hair
The complex interplay of estrogens and androgenic hormones in pregnancy leads to increased hair growth over the face (Figure 3) and, in some women, over the breasts and extremities as well. Estrogen prolongs the anagen (growing) phase of the hair cycle, leading to fuller scalp hair. The abrupt fall in estrogen postpartum precipitates a transition to the telogen (shedding) phase, resulting in hair loss termed telogen effluvium, that begins four to twenty weeks postpartum and can last up to a year. Return to pre-pregnancy hair growth usually occurs within 15 months (4).
-
Vascular
Spider angiomas — these lesions are characterized by a central red punctum with radiating branches and they appear on the upper body — usually develop in the first two trimesters of pregnancy. Palmar erythema localized to the thenar or hypothenar eminences may also be seen. Both these lesions occur in two thirds of Caucasian pregnant women, and are much less common in African-American and Asian women. The etiology is linked to estrogen (which has dilating effects on the spiral arterioles) and angiogenic factors, and the lesions usually resolve postpartum (6). Spider naevi in a unilateral linear distribution over the dermatomes of the face and neck, referred to as unilateral naevoid telangiectasia, has also been described in pregnancy (7).
-
Glandular
Estrogens have also been reported to suppress sebaceous gland function and may improve acne.
-
Connective tissue
Striae gravidarum develop in up to 90% of pregnant women and often resolve. Mechanical stretching of the skin in association with hormonal factors like estrogen, relaxin and adrenocortical hormones, which increase ground substance between collagen bundles in the dermis and decrease adhesive forces, results in the formation of striae distensae (4).
Pathological
The most commonly encountered cause of estrogen excess is pharmacologic or exogenous use of estrogens for either contraception, treatment of dysfunctional uterine bleeding or hormone replacement therapy (HRT). Other causes of estrogen excess include tumors of the ovary, testis, adrenals or hypothalamus, and cirrhosis of liver.
The appearance of circulating estrogens in prepubertal females results in precocious puberty. This may be gonadotropin independent, where there is administration of exogenous sex steroids or autonomous secretion of sex hormones from follicular cysts, adrenal tumors, ovarian tumors, or it may take the form of McCune Albright syndrome, a clinical triad consisting of precocious puberty, café au lait spots and fibrous dysplasia of bone, due to a mutation of the GNAS1 gene resulting in constitutively activated G-protein coupled receptors. Central or gonadotropin-dependent precocious puberty, on the other hand, is due to activation of the hypothalamus-pituitary-ovarian axis by hypothalamic or pituitary lesions.
In males, elevated levels of circulating estrogens occur in testicular feminizing syndrome (TFS) and in the presence of estrogen-producing tumors of the adrenal gland or testes. TFS is the result of a failure of testosterone and dihydrotestosterone to bind with androgen receptors. The absence of androgen effect is compounded by peripheral conversion of testosterone to estrogen.
Cutaneous manifestations of estrogen excess due to pathological conditions
-
Pigmentation
Melasma may be seen in up to one third of women taking an oral contraceptive pill (OCP). It usually fades within a year of discontinuation and may occasionally persist. Melasma can be aggravated by exposure to estrogens. Darkened (pigmented) areolae differentiated precocious puberty from transient neonatal breast hypertrophy due to transplacental estrogen in an infant girl with congenital hypothalamic hamartoma (8). Acanthosis nigricans can be associated with estrogen excess.
-
Glandular
Use of estrogen-dominant oral contraceptives can lead to improvement of acne as a result of inhibition of sebum production by estrogen.
-
Vascular
Patients taking synthetic preparations of estrogen can be prone to side effects such as telangiectasia, palmar erythema and spider angiomas. These may also occur as a result of reduced hepatic metabolism of estrogens, as in cirrhosis or alcoholic liver disease. Estrogen has also been linked to Raynaud’s phenomenon, the suggestion being that it increases the expression of α2C-adrenoceptors, which contribute to cold-induced constriction of cutaneous arteries (9). Associations have been seen between Raynaud’s phenomenon and unopposed estrogen therapy in postmenopausal women (10).
-
Breasts
Familial hyperestrogenism is a rare clinical condition characterized by increased conversion of C19 androgens into estrogens. Patients with this condition, also termed aromatase excess syndrome, presents with prepubertal gynecomastia, hypogonadism and short stature in men, and isosexual precocious puberty, macromastia and menstrual irregularities in women (11). Evaluation of a child with precocious puberty should include measurement of serum FSH, LH and testosterone in boys or estradiol in girls, and imaging to rule out central nervous system lesions.
-
Hypersensitivity
Autoimmune estrogen dermatitis due to hypersensitivity to estrogens has been reported. It is characterized by papulovesicular eruptions, eczema, urticaria and pruritus. These lesions may be generalized or localized to the genital regions (vulvar/anal) or the neck, upper arms and trunk, where there are dense estrogen receptors. The hallmark of this condition is the cyclical premenstrual flare. In severe cases, sex hormone allergies can even lead to anaphylaxis. The development of this condition has been linked to pregnancy, intake of exogenous estrogen or OCPs and in vitro fertilization procedures (12). The diagnosis may be made by administration of oral estrogen, but a positive test would indicate an estrogen aggravated dermatitis. Intradermal skin tests are necessary to show true sensitization. Persistence of a papule for more than one day after injection of an sterile aqueous preparation of estrogen is a positive result (2).
-
Dermatoses of pregnancy
Pemphigoid gestationis is a rare autoimmune bullous disease that occurs during pregnancy and the puerperium. It typically occurs in the second or third trimester, manifesting as pruritic papules, urticarial plaques, vesicles or tense bullae on an erythematous or normal base and it recurs with subsequent pregnancies, in which it appears earlier and in a more severe fashion.
20–50% of women develop recurrence of pemphigoid/herpes gestationis if treated with hormonal contraceptives (estrogens or progestogens) (13). Recurrence may occur within days to weeks after initiation of oral contraceptive use and the condition usually disappears after withdrawal of the treatment.
Intrahepatic cholestasis of pregnancy (ICP) is a pruritic disorder influenced by a genetic predisposition interacting with the effects of estrogen and progesterone metabolites on bile secretory mechanisms (14). Historically, ICP has been associated with the cholestatic effect of estradiol metabolites, particularly 17β estradiol glucuronide. Predisposed women often suffered from pruritus when taking oral contraceptives.
-
Skin disorders
Erythema nodosum
Erythema nodosum is a reactive inflammation of subcutaneous fat secondary to a wide variety of underlying conditions. Currently oral contraceptives and HRT are the medications most likely to induce erythema nodosum. The condition can occur de novo in pregnancy. Taken together, the association with oral contraceptives and HRT, the predominance of the condition in young women, and the occurrence of erythema nodosum in pregnancy suggest that estrogens may predispose to its development (15).
Porphyria cutanea tarda
Porphyria cutanea tarda (PCT), the most common human porphyria, which is often sporadic, is due to an acquired deficiency of the hepatic uroporphyrinogen decarboxylase (UROD) enzyme. PCT is often precipitated by alcohol consumption, hepatitis C and the use of estrogen, which increase the production of reactive oxygen species in hepatocytes, leading to oxidative changes that inhibit UROD.
Use of exogenous estrogen (oral contraceptives, postmenopausal hormone replacement and tamoxifen) has been found in up to 66% of female PCT patients (16). Infrequently, PCT has also been stimulated by pregnancy and childbirth (17).
Pellagra
Cutaneous manifestations of pellagra include glossitis, stomatitis, pruritic eruptions, erythema, slight hyperpigmentation and desquamation. Low plasma levels of pyridoxal 5’-phosphate,
the biologically active form of vitamin B6 (18), have been found in women of reproductive age and in users of oral contraceptives, linked to the competitive inhibition of kynureninase by estrogen metabolites (19).
-
Weight gain
High estrogen levels are associated with excess weight around the hips, thighs and lower abdomen.
Treatment
Triple ingredient preparations, consisting of a combination of topical retinoids, hydroquinone and corticosteroid bleaching creams, have been recommended as first-line therapy for treating melasma (20). Use of dual or single agents are second-line alternatives. If refractory, chemical peels (glycolic acid/ tricholoroacetic acid) can be tried. Lasers are of limited use in treating melasma. It is important to emphasize the concomitant use of sunscreens as melasma is exacerbated by sun exposure.
Licensed treatments for hirsutism include OCPs containing cyproterone acetate (anti-androgenic), cosmetic measures (such as laser, electrolysis, bleaching, waxing and shaving) and topical facial eflornithine.
Tamoxifen treatment of autoimmune estrogen dermatitis has been shown to be successful (21). The condition is likely to go into remission ultimately.
Gonadotropin-independent precocious puberty due to a tumor is treated, whenever possible, with surgery. McCune-Albright syndrome is treated with aromatase inhibitors (letrozole, anastrozole), tamoxifen, estrogen receptor antagonists (fulvestrant) and, in recalcitrant cases, with cystectomy or oophorectomy (22).
Familial hyperestrogenism is treated with second-generation aromatase inhibitors in both sexes. In addition, dihydrotestosterone is an efficient alternative in men with hyperestrogenism (11).
Effect of estrogen excess on other skin /connective tissue disorders
Pregnancy and the use of high-dose OCPs have been linked to improvements in psoriasis, while worsening of symptoms has been found with the onset of menses and menopause (23). In a study comparing 47 pregnant women with 27 controls (non-pregnant women with psoriasis), clinical improvement was noted in 55% of the women during pregnancy. 65% worsened postpartum. High estrogen levels, especially a high estrogen-to-progesterone ratio, have been associated with improvement of psoriasis. Progesterone levels did not correlate with psoriatic change (24). Impetigo herpetiformis, a rare pustular form of psoriasis precipitated by pregnancy, was reported to recur in nine successive pregnancies in one young woman. The condition was also precipitated by OCP intake (a combination of ethinyl estradiol and progesterone) in the same patient (25). Evidence on the effect of estrogens in systemic lupus erythematosus (SLE) is inconclusive. SLE may flare or present in pregnancy (2). A few studies have observed that OCPs can be used safely in patients with SLE in whom the disease is stable (26).
Estrogen deficiency
The etiology of estrogen deficiency can either be physiological or pathological.
Physiological
Estrogen deficiency is seen most often as a result of menopause, which occurs on average at about 50 years of age. The relative hypoestrogenism that accompanies menopause plays a key role in skin aging homeostasis as shown by the accelerated decline in the appearance of the skin that occurs in the perimenopausal period (27,28).
Pathological
Other less common causes of estrogen deficiency include pituitary/gonadal failure, exercise-induced amenorrhea, drugs such as danazol, leuprolide and clomiphene citrate that can induce a pharmacologic menopause, and surgical menopause due to removal of the ovaries. Girls with Turner’s syndrome have estradiol levels that are significantly lower than average and develop premature ovarian failure.
Cutaneous manifestations of estrogen deficiency
-
Skin
Estrogen deprivation accelerates the xerosis, atrophy, wrinkling and poor wound healing that accompanies cutaneous aging. These changes are due to structural alterations in the skin, such as decreases in collagen content, dermal thickness and elastin fibers, and they are reversible with the administration of local or systemic estrogen (29,30). The loss of dermal collagen has been found to be inversely related to the number of years since menopause, regardless of the patient’s age, while it parallels bone loss. Estrogen also retains skin moisture by promoting sebum production and improving the water-holding capacity of the stratum corneum (31), an important contributor to the barrier function of the skin. Treatment with estrogen increased the hyaluronic acid content of skin in mice, which closely correlated with an increase in tissue water (32).
Fluctuating estrogen levels in the perimenopausal and early menopausal period bring about vasomotor symptoms called hot flashes in 75% of women. These are thought to arise from dysregulation of temperature-controlling regions of the hypothalamus, triggered by declining estrogen levels, coupled with an increased peripheral vascular reactivity in symptomatic women (33). The hot flash is due to cutaneous vasodilation and increased blood flow to the skin, resulting in erythema and sweating. This is often localized to the head, neck and trunk rather than the periphery, as is instead the case with exercise, suggesting a different mechanism. The correlation between hot flashes and declining estrogen levels is not clearly understood but is supported by the effectiveness of estrogen replacement therapy.
-
Genitourinary system
The lower urinary and reproductive tracts share a common embryonic origin and estrogen receptors (34). Estrogen maintains the acidic nature of the vagina which provides an innate defense. Loss of estrogen is responsible for an altered vaginal pH >5, which leads to replacement of the healthy lactobacillus-predominant flora with gram-negative rods leading to vaginitis and urinary tract infections. Loss of mucosal vascularization results in vaginal and urogenital atrophy. Estrogen receptors in the bladder trigone and urethra maintain sensory thresholds during bladder filling. The loss of estrogen after menopause results in lower leak-point pressures and urgency (35). This constellation of progressive atrophy of the vulvovaginal and lower urinary tracts is termed the genitourinary syndrome of menopause.
Vulvodynia
Vulvodynia is defined as painful sensation of the vulva in the absence of clinical or laboratory abnormalities. The pathogenesis is multifactorial and one of the factors implicated is inadequate estrogen effects.
The onset or worsening of this pain has been linked to times of estrogen loss, such as menopause and postpartum (36). Studies have shown a decrease in estrogen receptors of the vulva in this condition and estrogen deficiency may result in an increase in local nerve density.
Labial adhesions
Superficial fusion of the labial surfaces occurs in 1–3% of prepubertal girls, usually at 2–3 years of age. Lack of estrogen may play a role (37) in the inflammation and adherence of labial skin surfaces. This condition usually involves the posterior two thirds of the labia minora and, rarely, total fusion may occur. It usually resolves spontaneously at puberty when estrogenization occurs. The first line of therapy is topical estrogen cream, but if the condition is extensive, surgery may be required.
Vulvar dermatoses
The vulva is relatively estrogen deficient between 3 months of life up to the 3 years before menarche. Many childhood vulvar problems are related to lack of estrogen. The vulvar epithelium and labia minora are thin with a visible capillary network and are susceptible to irritants but resistant to candida. Estrogen is an important factor in vulvar defenses against irritants and infection (2). Postmenopausal women show a decreased resistance to infections, which results in recurrent candidiasis etc.
-
Wound healing
Declining skin elasticity leads to fragility and easy susceptibility to trauma and bruising (38). Fibronectin plays an essential role in wound healing, by directing keratinocyte migration and fibroblast proliferation and stimulating collagen deposition and wound contracture. It is degraded by elastase, a proteolytic enzyme produced, at the wound site, by neutrophils, which are found in greater numbers in postmenopausal women. Estrogen inhibits the chemotaxis of neutrophils to wounds, preventing fibronectin degradation and accelerating wound healing (39).
-
Hair
Female pattern hair loss (FPHL) or androgenetic alopecia (characterized by thinning of hair at the vertex with preservation of the frontal hairline), is caused by an alteration of the hair cycle, due to a shortening of the anagen phase and prolongation of the telogen or shedding phase. It is characterized by gradually replacement of the terminal hair of the scalp with fine short vellus hair (40). This is hypothesized to be due to an increased androgen sensitivity, and is known to occur in women with other signs of hyperandrogenism like acne and hirsutism. Estrogen exerts a protective role against hair loss by inducing aromatase activity in scalp hair follicles. Women with relative estrogen deficiencies, such as during menopause, postpartum or treatment with aromatase inhibitors or selective estrogen receptor modulators, are more likely to develop FPHL (41).
-
Nails
While changes in the appearance of nails, such as brittle nails, can occur for a variety of reasons, the most likely cause in women of menopausal age is estrogen deficiency. Low estrogen levels can cause dehydration leading to brittle nails.
Treatment
Estrogen replacement therapy has been shown to increase the thickness and water-holding of the skin, to increase the lipid composition of the stratum corneum, and improve skin laxity (42). Wound healing has also been shown to improve with estrogen treatment (43). Administration of estrogens in postmenopausal women can reverse epidermal atrophy and increase cutaneous collagen. But the risks and benefits of HRT must be weighed up on an individual basis. Vaginal administration of estrogen is more efficacious to treat genitourinary atrophy. The immature atrophic mucosa is often transformed in 3–4 months. Low-dose clonidine, paroxetine or venlafaxine can be considered to treat menopause-related hot flashes when hormone therapy is contraindicated, although it is less effective. FPHL can be arrested using anti-androgen therapy such as spironolactone, cyproterone acetate, the 5a-reductase inhibitor finasteride and an androgen-independent hair growth stimulator minoxidil. Estrogen-containing oral contraceptives are also effective.
Effect of estrogen deficiency on other skin /connective tissue disorders
Symptoms and signs of lichen sclerosis are aggravated by estrogen deficiency in postmenopausal women and prepubertal girls (2). Postmenopausal women benefit from the use of estradiol cream 1 g inserted in the vagina three nights a week.
Conclusions
Estrogen has a profound influence on the skin. Specific diseases appear as a consequence of hypo or hypersecretion of estrogen. Knowledge of skin alterations is important not only for dermatologists, but also for endocrinologists and physicians because a clinical diagnosis of the underlying disease is often possible.
Conflict of Interest Statement
None declared