Introduction
Diapause, a survival mechanism that represents an intermediate step between fertilization and embryo implantation, is reported to occur in >130 mammalian species. Delayed implantation extends gestation duration in 70% of eutherian and ~30% of marsupial species 1. Although the embryo transits and reaches the uterus, it does not implant. The length of the implantation delay depends on the season, food availability or the photoperiod. For example, the European roe deer embryo enters diapause at day 14 post fertilization, from August to December; the subsequent implantation and pregnancy take up to 9 months2. In multi-litter rodents, energy partitioning favors currently lactating pups until weaning, while maintaining the other embryos in diapause 3. Moreover, in marsupials, lactation reversibly arrests the upcoming embryo that is ready to implant. The regulatory compounds responsible for progressing/stopping the embryo’s metabolic state vary 4. The embryo’s ability to stop/resume development is innate and depends on need. Regardless of whether the diapause mechanism is obligate or facultative, embryo-maternal dialogue is effective. When the maternal milieu is right, i.e., permissive, pregnancy will progress until term. The ability of the maternal endometrium to recognize and enable embryo development during the implantation window (IW), creating a suitable environment, is under the control of estrogen (E2)/progesterone (P4). Whether diapause can be deliberately delayed due to internal or external influences is not fully elucidated.
With the advent of ART/IVF, it became possible to separate the embryo’s fate from that of the mother/surrogate. Each party is examined individually until embryo transfer (ET) when embryo-maternal dialogue becomes direct and intimate. We aim to describe innate embryonic diapause mechanisms in vivo, and autonomous cultured embryo development and survival as nutrients are supplied. In parallel, we analyze the endometrial milieu both pre-priming and in diapause, differentiating between endometrial receptivity and embryo development pre/post transfer. Finally, we look at whether coordination of embryo and endometrium is assessed during diapause. ART is still evolving, and the take-home baby rate is still only ~30%. Therefore, lessons learnt from this ancestral successful reproductive modality — diapause — could elucidate causes of failures and improve reproduction in human and non-diapause mammals5.
Biological rationale for mammalian diapause
Present in insects, crustaceans, and fish species, diapause is an integral part of evolution, since delayed development supports offspring survival. The crustacean Artemia sinica thrives under hypersaline water conditions, but under hypothermic stress diapause occurs, with glycerol accumulation providing cryoprotection. When temperatures improve, the process of embryo development is resumed, with the stored glycerol serving as a glucose precursor and energy source 6. In summer, when ponds dry out, teleost fish survive thanks to their desiccation-resistant embryos, while all adults are eliminated. Post-diapause embryo hatching occurs in the next rainy season 7. As evolution advanced, internal rather than external aqueous fertilization appeared. The development of a post-fertilization embryo encased in a shell still amounts to development in an external environment. In reptiles, and to a lesser extent in birds, in-uterus and in-shell embryonic diapause can occur. In the latter case, the hard shell provides protection until hatching, independently of the internal maternal milieu. Depending on parental care, such embryonic diapause may be needed for survival. Reptile embryos may undergo diapause pre/post oviposition, ensuring their survival and hatching in the favorable season 8. Reflecting a biological imperative, phylogenetic diapause is present in >130 different mammalian species, both metatherian and eutherian, ranging from mice (ancestral) to recent deer and bears 9,10. Diapause is individual and depends on weather and food availability, critical for effective implantation. Moreover, delivery should coincide with spring, the most favorable season of the year, when offspring survival is highest.
Pre-implantation embryo developmental delay and maternal interactions have together allowed the perpetuation, to today, of certain species, ranging from marsupials to eutherian mammals. Early delicate dialogue is a must for implantation (receptivity) and, throughout the entire course of evolution, radical environmental and climate fluctuations have led to adaptation of diapause. Artic polar bears ovulate in spring, accreting fat (> 50% of body mass); implantation occurs during fall followed by hibernation through winter with parturition in December. The stored adipose tissue will be used efficiently through β-oxidation of fatty acids until the adult female and her cubs (~2) emerge from the den the following spring, returning to their favorable sea ice environment. By the end of this reproductive cycle, a total of 8-9 months of fasting is associated with ~40% body weight loss11.
For some unknown reason, diapause is not observed in primate and humanoid species10. And this is despite humans’ continuous adaptation to harsh climates and limited nutrition. In humans, an adverse environment impairs ovulation or prevents fertilization and may cause early subclinical loss 12. Similarly, tapered-head sperm or oligospermia reduce fertilization potential 13. Also, in humans, thanks to the use of contraceptive methods, births can be spaced at will, without diapause. ART has removed many obstacles to reproduction in mammals. Gamete and embryo collection coupled with cryopreservation has enabled species survival, with deliberate programming of pregnancy and parturition. One case report suggested the occurrence of diapause in a patient 7 years after tubal ligation. Following IVF, 3 embryos were transferred but bhCG was negative after 15 days. However, at 7w post ET, without menses, a low HCG level (329 IU/ml) was noted 14. Later ultrasound confirmed pregnancy and a healthy delivery followed. Here, however, tubal recanalization may possibly have occurred.
Embryo-maternal adaptations to the environment: reproductive hormonal milieu and pre-implantation embryonal factors
Hormonal control of embryonic diapause in utero has been shown in mice, marsupials and mustelids. While cycle length is narrowly fixed, the length of diapause may vary depending on the environment, climate, nutrient availability, and herd migration. Entry into embryonic diapause depends on fuel sensing regulation and energy partitioning towards a specific physiological state. For example, prolactin (PRL) release controls the ratio and action of sex steroids, P4 and E2, preventing implantation during lactation in order to ensure nourishment of the future offspring. Diapause lasts from weeks up to one year in certain marsupials like the tammar10.
Variations in diapause length are opportunistic, having a specific goal, namely blastocyst preservation until the optimal time arises. In marsupials, both E2/P4 levels drop, while PRL rises, due to the suckling effects of the small size embryo on maternal hair follicles, causing milk release. Thus, effective birth spacing is combined with adequate care for the embryo in the pouch. After the newborn is weaned, PRL levels drop to enable the next embryo to implant and migrate into the pouch 10. In humans, lactation, stress, and prolactinoma cause disorders ranging from anovulation to amenorrhea15. In mice, despite PRL-induced E2 reduction, levels of P4 (from the corpus luteum) remain elevated, priming the secretory endometrium to support diapause embryos. Embryo re-activation coincides with increased E2, necessary to stimulate the action of P4 through increased expression of the P4 endometrial receptors, thereby optimizing the endometrial milieu 9. Administration of bromocriptine, a dopamine agonist, stops the diapause phase in mice 16. In the seasonal reproduction of the mink, the long night increases melatonin secretion, which decreases E2/P4 and PRL levels leading to entry into diapause. Surprisingly, however, embryo re-activation also depends on PRL levels 10,17.
Pandas breed in the spring and cubs are born in the late summer/autumn. After mating/AI, diapause follows for several months, when P4 rises. Implantation and pregnancy start with a secondary P4 rise lasting 40-55 days. This rise occurs irrespective of outcome, i.e., pregnant, mated not pregnant, or not mated pseudo-pregnancy. Ceruloplasmin testing suggests potential conception and embryo presence in uterus at the secondary P4 rise. There are two prostaglandin F2α spikes, one 23-25 days before birth and second at 24 hours before delivery. Thus, pregnancy length is 30-42 days 18. Additionally, due to its small size the embryo can be detected by ultrasonography only ~15 days prior to parturition. The external signs of pregnancy, i.e., breast and vulva changes, and urinary peak of P4 are detected only after 100 days of gestation.
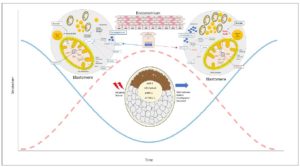
Several studies have identified uterine secretions and glandular activity that, through embryo-maternal dialogue, create different nutritional environments supporting diapause embryo welfare 10,19,20-23. Preimplantation factor (PIF) is an ancestral peptide secreted by viable embryos that is preserved from rodent, bovine, and other mammals up to humans 24-26. PIF exerts autotrophic and protective effects on the preimplantation embryo 27. It primes the secretory endometrium (better than P4), and both during and after implantation it promotes endometrial receptivity 28-30. Circulating PIF levels in early gestation correlate with favorable bovine pregnancy outcome 26. Expressed by trophoblasts, PIF regulates controlled trophoblast invasion and increases HLA-G and similar pro-tolerance molecules, as well as P4 and its receptors 31-34. PIF is secreted by viable embryos and added in culture prevents cultured murine embryo demise caused by serum of patients with a history of recurrent pregnancy loss or oxidative stress, while promoting blastocyst development 25,27. PIF administration from conception leads to a 3-fold reduction of spontaneous and LPS-induced murine fetal demise and a four-fold reduction of LPS-induced prematurity 35,36. Thus, a role for PIF during preimplantation and beyond is present both in species with and without diapause. Amino acid profiles vary during embryonic diapause 10. Glutamine uptake and metabolism along with beta-oxidation of fatty acids are upregulated. 19. Reduced dietary branched chain amino acid (BCAA) uptake in embryos may minimally contribute to the TCA cycle which downregulates mTORC2 activity. In turn, BCAA ,may upregulate lipolysis genes while downregulating protein synthesis 19. Furthermore, glycogenolysis from endometrial glycogen reserves may supply glucose precursors to support diapause embryo welfare while lactation is taking place in a season with limited food resources 21 (Figure 1).
Through remarkable cellular plasticity, both embryo and endometrium adjust in such a way as to mutually provide key elements for reproductive success. Having a dual supportive role, the endometrium also supplies nutrients for re-activated embryos. Major differences emerge when comparing diapause and activated endometrium. Beyond changes in E2/P4 levels, cytokines are also modulated. Post activation, an epidermal growth factor (EGF) pathway increases HB-EGF receptor expression and also binds the ERBB4 receptor on blastocysts, critical for both human and mouse implantation 37. Platelet-activating factor (PAF) targets the platelet-activating factor receptor (PTAFR) on embryos, while increased endometrial vascular endothelial growth factor (VEGF) expression promotes trophoblast vascularization. Notably, at peri-implantation, a lactate dehydrogenase (LDH) isoform switch may take place 38. Thus, the increased LDHB at diapause promotes formation of pyruvate, the main energy substrate. Post-diapause, LDHA, which favors lactate formation, is dominant and facilitates trophoblast invasion by upregulating angiogenic VEGF isoforms 38. The embryo-induced high lactate microenvironment may recruit macrophages that produce VEGF and, by immunomodulation, prevent T-cell-induced rejection by the mother 38. Leukemia inhibitory factor (LIF) is an E2-dependent factor, belonging to the IL6 family, that binds blastocysts through the LIF-gp130 receptor19. LIF-induced inflammation widens intercellular spaces, enabling effective embryo-maternal contact. A study of mink endometrium profiles comparing diapause vs. post re-activation identified 123 differentially expressed genes involving cell proliferation, immune response and factors affecting remodeling 23. The polyamine pathway effect on the uterus and embryos is marked, whereas during diapause it is reduced 9. Also, FoxO1 transcription factor involved in the insulin signaling pathway is down-regulated in diapause partly through a limited to null BCAA contribution, which lowers the AMPK-mTOR-FoxO1 pathway activity 20, 39. Post diapause, re-activation occurs rapidly at the local level without obvious changes in systemic hormone levels, which occur later. Thus, embryo re-activation is due to local factors involving both the embryo and the endometrium that becomes receptive. Overall, many synergized ligands/pathways are needed to support endometrium flexibility and reversibility to sustain the embryo until delivery.
Despite the diversity of mammals, the same hypothalamus-pituitary-gonadal axis (HPG) hormones are in play, but their secretion and action occur with different timing. Considering that >130 mammalian species and eight different orders undergo diapause, different patterns of the same hormones are to be expected. Through an orchestrated embryo-maternal process, re-activation (re-initiation of implantation) occurs at ~16h in all species studied, supporting embryo rapid adaptability 40. ART has made it possible to demonstrate innate survival mechanisms of gametes and embryo development during in vitro culture followed by opportune time to implant post-ET leading to successful pregnancy outcome. However, ART is not always successful and a pregnancy that follows ET is considered high risk 41,42; therefore, this still evolving art might benefit from improved understanding of how diapause enables prompt intimate embryo-maternal synergetic communication and from identification of the specific factors involved43. Viable embryo-derived signaling could be part of the solution since without PIF there is no pregnancy 26.
Embryo semi-autonomy as achieved during diapause is long lasting in culture
Post-implantation embryo survival can be prolonged in diapause mammals compared with humans. By contrast, gametes have a limited life span unless fertilization occurs within 12-24 hours 44. Egg maturation in vitro makes it possible to expand their life span and fertilize them in culture 45. However, the zygote, through innate properties, contains the basic building blocks of life to form a whole organism. Furthermore, the embryo’s ability to implant beyond the endometrium at different sites (fallopian tube, ovary, and abdominal cavity) proves its resilience and adaptability to diverse and even adverse environments.
To better understand diapause, ART documents embryo autonomy, and the length of embryo survival without any maternal influence. IVF resembles oviparity, in the sense that, after eggs are fertilized in culture, the embryos develop independently of their biological origin and totally depend on the surrounding environment i.e., embryo culture system. Additionally, before we moved beyond the in vivo ART era, separating and analyzing embryo-maternal crosstalk was difficult. Current culture methods and more defined media prototypes now enable investigators to closely observe embryo morphokinetic development through precision technology 46. Cryopreservation proved that metabolic activity of both sperm and eggs can be halted and preserved indefinitely. Analogously, embryo cryopreservation closely mimics diapause since an embryo may survive long term without apparent damage. During diapause, a blastocyst stage embryo in the endometrium can stay dormant for up to a year, remaining viable until a favorable maternal environment is present. Therefore, prolonged dormancy can be successfully reversed to achieve pregnancy. Cryopreservation causes total developmental arrest and no metabolic activity, while diapause embryos are alive, albeit with reduction of their metabolic activity and profile 19. Whether, instead of cryopreservation, a gradual embryo preservation method would benefit ART is unknown. Therefore, examining the longevity of cultured embryos under adjusted metabolic activity may be informative and should be pursued. Human embryos are cultured until day six reaching an advanced blastocyst stage at which it is possible to assess morphology, genetic quality, and specific viability markers. However, human embryos can develop in culture for up to 14 days 47. In murine IVF, both short- and long-term embryo culture increased cardiovascular or metabolic disorders, suggesting that further improvements in culture methods are needed in order to reduce these risks since, overall, pregnancy after ART is considered high risk 48-50. In rodent embryos under adequate nutrient supply and culture conditions in roller bottles, development was supported up to the stage when a heartbeat was already present 51. This means that embryo implantation in the uterus is obligatory only for a short period, since prematurely born 22w babies can survive under intensive support 52. Overall, the pre-implantation embryo’s ability to develop, stop and start again is innate and independent of maternal influences. Moreover, blastocysts reaching the endometrium retain semi-autonomy despite the minimally supportive maternal environment. Therefore, without maternal prior information embryos must be ready within ~16hrs to re-activate, showing metabolic activity, shedding the zona pellucida, and resuming development. Such findings reflect highly conserved embryo off/on innate metabolic mechanisms. In mice, facultative embryonic diapause may occur at day 3.5 of development, but the timing and length may also be flexible.
Mechanistically, embryonic diapause is regulated by an Lkb1 gene splice variant. The long form of this kinase alone can downregulate AMPK and consequently mTOR pathways causing embryonic diapause. This is coupled with increased glycolysis, lipolysis, and glutamine uptake by the embryo 19. Moreover, reduced cell proliferation is caused by downregulation of mTORC2-mediated allosteric activation of the pentose phosphate pathway (PPP). The rate-limiting enzyme G6PD decrease keeps the ICM cells in a dormant state. Indeed, regulation of mTOR signaling on the PPP through steroid receptors was also reported in cancer cells 53. Therefore, embryos exit diapause with resumption of the mTORC2 pathway, and upregulation of PPP to promote cell proliferation. Additionally, a potential link between PPP and IFNτ expression and initiation of maternal recognition is observed in bovine species. Moreover, dimorphic metabolic differences on the sex chromosomes have been observed 54,55. This supports innate embryo flexibility in rapidly responding to drastic changes present in the surrounding environment. As diapause requires a gradual entry into a different metabolic state, it adds complexity to the bidirectional embryo-endometrium communication (Figure 1). Thus, we postulate that the embryo in that respect is opportunistic, acting as a parasite aiming to further its own development regardless of how challenging its surrounding environment is. A better understanding of embryo flexibility before/during after diapause could improve ART and reproduction in general.
Following conception, embryo transfer or diapause, the blastocyst reaches the endometrium
Irrespective of reproduction modality, sperm and egg must interact to form the zygote followed by the morula up to the blastocyst stage. Post conception, as embryos reach the uterus, the maternal environment starts to adapt, primed by E2/P4 and by specific signaling such as PIF reported in mouse, bovine, and human embryos 24. Thus, both with spontaneous conception and fertility therapy, the blastocyst, to implant, must reach the already primed uterine cavity. Moreover, in modern ART, zygote intrafallopian transfer has been replaced with less invasive techniques such as blastocyst transfer 56. Long-term culture of 5-6 days allows it to be determined, through a process of self-selectivity, which embryos are to develop. Once the blastocyst stage is reached, the post-ET pregnancy rate is ~50%; however, it is followed by a take-home-baby rate of only ~30%. Overall, in scenarios such as natural conception, ET, and diapause, similar success rates are present. Biologically this is a great advantage, since if most defective embryos were to implant, species would not survive the consequent repeated losses of progeny despite intensive maternal efforts. Post-implantation, an additional 15-20% of early pregnancy loss occurs; while this is of limited importance in poly-ovulatory species, it is critical in the mono-ovulatory human species.
Maternal recognition of pregnancy following spontaneous conception and diapause may occur post fertilization, but it will be short lived if embryos do not develop. By contrast, embryo-driven signaling, which promotes endometrial receptivity, is not operative until post-IVF ET. Endometrial priming through E2/P4 is required to create a favorable secretory-type decidua lining, thereby enabling implantation of a transferred/donor embryo 57.
In humans, maternal recognition of pregnancy through embryo-specific signaling occurs within 3-4 days after ET when embryo-maternal contact is direct and intimate. This occurs irrespective of the circumstances: natural conception or fertility management. The limitation is that embryos need to reach the endometrium during the narrow IW, i.e., the period of synchrony between embryo and endometrium. Delayed or premature ovulation may affect embryo quality; this and/or altered endometrial health can cause asynchrony, where even 2-3 days deviation in the IW may cause implantation failure. Thus, both an embryo of quality and a receptive and healthy endometrium are required for reproductive success. In bovine species, the delay between conception and implantation is five time longer than in humans, even though they show a similar gestation length, of 270-280 days 58. Consequently, by the time of implantation, bovine blastocyst development will be advanced. Irrespective of the species-specific delay in blastocyst formation and effective implantation, diapause is an independent phenomenon 59. Although endometrial priming will start post-fertilization as in vivo, in diapause it will not be amplified or completed since initially only embryo apposition will take place. In ART, after apposition, embryo hatching, adhesion and extravillous trophoblast invasion will promptly follow, since failure of any step will cause embryo demise. In diapause, the next developmental steps beyond apposition will only take place after re-activation. However, all reproductive modalities require intense and progressive maternal adaptation to pregnancy. For the mother, investing significant resources if pregnancy is likely to fail would be a great disadvantage. Therefore, following successful implantation, and considering pregnancies after the first trimester, the take-home-baby rate, at least in humans, is close to 100%, unless maternal or fetal disease occurs.
Embryo quality assessment upon reaching the uterine cavity is variable. For example, following spontaneous conception or ET in species without diapause, pregnancy resolution is rapid and faulty embryos either do not implant or are quickly eliminated peri/post implantation, this being followed by menses in humans or estrus in mammals. In diapause, the blastocyst is next to the endometrium but does not implant; this allows intimate quality assessment, while requiring minimal maternal adaptation. This embryo-maternal communication is amplified both by secretory products and by direct contact and can last for several months, enabling effective decision processes when re-activation is opportune.
Compared to the narrow IW in humans, during diapause embryo quality is continually scrutinized; this is followed by re-activation as pregnancy advances. The timing is highly variable depending on external influences, climate, season of the year and availability of nutrients. Notably, diapause is rare in tropical climates where the seasons are stable. However, at higher altitudes, where low temperatures, snow and storms can develop, a similar adaptation strategy may be seen 59. In different species maternal adaptation is thought to be highly variable, aided by continuous embryo-maternal communication enabling rapid re-activation followed by uninterrupted development until term. It is not known whether environmental changes following embryo re-activation further extend semi-dormancy, necessitating further re-activation.
A recent study offered significant insight into diapause as a dynamic process. Sheep IVF embryos transferred to the mouse uterus entered a diapause state 59. Subsequently embryos were recovered and transferred to the sheep uterus, where blastocysts implanted and led to healthy pregnancy outcomes and progeny. Thus, cross-species transfer is successful, genetics does not play a role, blastocysts can sojourn without any adversity in surrogate/xeno endometrium, and when transferred to the sheep good pregnancy outcomes ensued.
What remains unclear is whether diapause for a given species is innate or represents adaptation to need. Also, it is not known whether entry or non-entry into diapause is a fixed-timed process that, once established, does not change, or whether it is a dynamic and species-specific process. A further question is whether diapause can be lengthened or shortened dependent on maternal conditions and/or surrounding events. In the future, it would be interesting to study whether more species enter a diapause state as the climate and environment continues to change. Overall, diapause is effective, enabling dynamic multistep embryo-maternal cooperation that leads to favorable pregnancy outcomes. Better knowledge on how to enhance the maternal milieu to enable effective embryo re-activation in the presence of favorable embryo development could improve both ART and reproduction. 60
With regard to the issue of interaction between fertilization and the prospective mother, progress has been made through the use of a capsule for fertilization. This method entails intimate and close contact between sperm and oocytes followed by intravaginal incubation.61 Based on current data, this method of IVF achieves success rates similar to those of traditional IVF. Whether such intrabody-based incubation also provides continuous embryo-maternal communication is not clear since the method is not superior to traditional IVF in terms of success rates. However, due to its reduced cost, simplicity and current patient acceptance, this reproduction mode will likely become widely used. What it still lacks is the intimate embryo-maternal contact/communication that is observed in diapause. Overcoming this aspect may further increase the ART success rate.
Conclusions
Nature through the course of evolution had to devise complex and highly effective reproductive mechanisms for species ranging from invertebrates to mammals, enabling separation between mating, embryo development, maternal acceptance, and pregnancy. Diapause entails pregnancy and delivery at an opportune time and in favorable surroundings. The fact that this multistep mechanism still persists in both marsupials and >130 different mammalian species proves how critical it is this for the survival of given species. The extinction of various species may have been caused by failure to adapt to this sophisticated mechanism. In diapause, embryo autonomy with regard to quality-based self-selectivity and ability to survive during semi-dormancy must be present. Intimate effective and precise embryo-endometrium contact, and communication enable embryo re-activation from quasi-starvation for a year to full activity, which is remarkable.
The advent of ART has enabled us to replicate diapause only partially. Sperm and eggs can be cryopreserved almost indefinitely, fertilization occurs in vitro, at any time, and embryo self-selectivity has been shown to occur, since most low-quality embryos do not reach the blastocyst stage. Cryopreserved embryos are stored long term and, post thawing, they are transferred within hours to the uterus. Thereby implantation timing can be precise and if pregnancy ensues its eventual outcome can be decided in advance. Obviously in humans, the timing of IVF and ET is based on individual choice and not on the need to preserve or improve reproduction as in mammals. Despite ART, IVF/ET still show major limitations, for which diapause may offer some solutions. Currently, embryos with apparent normal morphology often do not implant following ET. This may reflect an innate embryo and/or maternal defect. Thus, improved culture milieu and/or added trophic factors may improve outcomes. Further, the question of whether the use of embryo semi-dormancy instead of cryopreservation may be preferable has not been tested. Gradual re-activation to prevent a drastic shift from arrested metabolic activity may be favorable. Such a gradual approach would enable innate trophic mechanisms to be awakened and improve embryo signaling to the mother as observed in diapause. The second aspect, related to the endometrium in species with diapause, is that the delay between apposition and embryo re-activation is maternally driven. Currently post-ET endometrial adaption may be less than optimal since it consists of a rapid yes/no decision. In diapause, on the other hand, there is prolonged and sustained embryo-maternal interaction. Better preparation of the endometrium through embryo-specific signaling systems like PIF may amplify the efficiency of the IW, which may be deficient at present. In a certain sense diapause is a gentle and progressive interaction between two different (semi-allogenic) entities — the embryo and the mother — acting for mutual benefit and success. Overall, lessons learnt from this effective ancient mode of reproduction could improve ART and overall reproduction success in both human and other non-diapause mammals.
Declaration of Interest:
Eytan R. Barnea, MD FACOG discovered and developed the PIF-based technology completed phase I fast-track FDA approved first in human clinical trial. I (E.R.B) am the immediate past CSO of BioIncept, LLC and together with my wife, Dr. Jacqueline Barnea, LLD (immediate past CEO) we own in trust minority shares.