What is kisspeptin?
Kisspeptin is a peptide encoded by the KISS1 gene and it was first identified in 1996 (1). The first studies on kisspeptin were conducted in the field of oncology, where the KISS1 gene was first discovered and found to be expressed in malignant melanoma cells. Kisspeptin was originally known as "metastatin" on account of its ability to inhibit the metastatic process (2). Both the gene and the peptide were later named kisspeptin after the most famous local product on the university campus in Hershey, Pennsylvania, where the gene was discovered, namely the famous chocolates known as "Hershey's Kisses". Interestingly, a few years after its discovery, kisspeptin turned out to be a hormone that plays a key role in reproduction (1).
Kisspeptin acts by binding to the G protein-coupled receptor 54 (GPR54) also known as the KISS1 receptor (KISS1R). This receptor, which belongs to the family of G proteins, consists of seven transmembrane domains and its sequence it is similar to that of the galanin receptors. Both kisspeptin and its receptor, GPR54, have so far been identified in fish, amphibians, birds, rodents, sheep, horses, monkeys and humans (3).
The KISS1 gene is localized to chromosome 1q32 and has four exons, the first two of which are not translated. The initial protein product of the KISS1 gene is a 145 amino acid prohormone, kisspeptin 145 (KP-145), which undergoes proteolysis. By proteolysis, it is cleaved into shorter, biologically active peptides known as kisspeptin-54, kisspeptin-14, kisspeptin-13 and kisspeptin-10, each number representing a number of amino acids. These activate the protein receptor GPR54, which, bound to the G protein, is the receptor for the peptide products of the KISS1 gene (4).
The reproductive role of kisspeptin
Regulation of GnRH
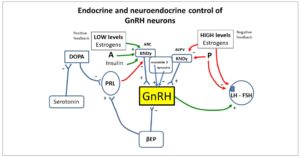
The importance of the KISS1/KISS1R system in reproduction was discovered in late 2003, when KISS1R mutations were first detected in humans and mice suffering from hypogonadotropic hypogonadism (5, 6). Kisspeptin was soon located at a superior level of the reproductive axis. Specialized hypothalamic centers (kisspeptin neurons) have the ability to secrete kisspeptin. They are located in the rostral preoptic area and the infundibular nucleus in the human hypothalamus (Fig. 1). Kisspeptin has proven to be one of the strongest stimulants of gonadotropin-releasing hormone (GnRH) secretion in various mammals, including humans (7).
Kisspeptin induces the expression and modulates the function of kisspeptin receptors on GnRH neurons, thereby stimulating the sex hormone-mediated secretion of gonadotropins, and it regulates fertility. Additionally, kisspeptin neurons respond to many other hormones and neuropeptides, including vasopressin, oxytocin, prolactin, leptin, pro-opiomelanocortin, neuropeptide Y, GABA (γ-aminobutyric acid), glutamate, nitric oxide, neurokinin B, dynorphin and dopamine; kisspeptin is thus at the heart of a complex network, coordinating reproductive, metabolic and behavioral reproductive control (8).
Furthermore, in addition to kisspeptin-expressing neurons, neurokinin B (NKB)- and dynorphin (DYN)-expressing neurons are also present in the arcuate nucleus (ARC) of the hypothalamus. Together, these are known as the KNDy neurons, from the initial letters of the names of their neuropeptides (kisspeptin/neurokinin B/dynorphin) (Fig. 1). GnRH secreted in the hypothalamus plays a major role in the regulation of the hypothalamic-pituitary-ovary axis, and kisspeptin, NKB and DYN significantly influence the regulation of GnRH secretion (9).
Sexual dimorphism
There exists a lot of evidence confirming that the kisspeptin pathways in humans show sexual dimorphism. Women, for example, have significantly more kisspeptin fibers than men in the infundibular nucleus and ventral periventricular zone of the hypothalamus. Furthermore, a huge gender difference is evident in the number and expression of kisspeptin cell bodies, which only in females are seen in the rostral periventricular zone. Additionally, in women a huge presence of kisspeptin cell bodies was observed in the infundibular nucleus, while in men only a few kisspeptin cell bodies were found. Moreover, only women have positive sex steroid feedback, which is not present in men (10).
Similar observations apply to animals, with the hypothalamus of adult female mice and rats containing 10 times more kisspeptin neurons in the rostral periventricular region of the third ventricle than were found in males. On the contrary, the ARC, which is responsible for the negative feedback of sex steroids, does not show such dimorphism (11).
Positive and negative feedback
In the follicular phase of the woman's menstrual cycle, GnRH activity and luteinizing hormone (LH) secretion are limited by negative feedback of estradiol. The end of the follicular phase in women is characterized by a change from negative feedback to positive, causing an increase in GnRH and LH secretion during ovulation. Although this neuromechanism is not fully understood, the KNDy neurons seem to be deeply involved (Fig. 1). Instead, positive feedback of sex steroids appears to be more species specific (12).
Recent data support a potential role of kisspeptin in generating the ovulatory LH rise in humans (13) and kisspeptin seems to be responsible for positive feedback (Fig. 1). However, the site of action of kisspeptin is different in rodents, humans, and other species. In rodents, the anteroventral periventricular (AVPV) nucleus is the site of estrogen positive feedback, while in humans, other primates, and sheep, the kisspeptin neurons are found in the ARC (also known as the infundibular nucleus) (14).
Kisspeptin and metabolic signals
The hypothalamus plays a key role in maintaining fertility in all mammals. The KNDy neurons in the preoptic area and the ARC control the secretion of GnRH, by neurons located in the median eminence, into the portal circulation of the hypothalamus and pituitary gland.
The KNDy neurons seem to be the main node connecting energy stress with reproductive functions. However, within the hypothalamic nuclei there are many other neurons with numerous surface receptors for peripherally secreted adipokines. These neurons, by affecting the KNDy neurons, have the ability to modulate reproductive functions.
Metabolic control at the hypothalamic level occurs in the ARC (15). Many neurons potentially involved in the control of metabolism are located in the ARC. They include POMC/CART neurons and AgRP/NPY neurons involved in the regulation of body weight and food intake. These neurons are known to communicate with kisspeptin neurons and while they express receptors for adipokines, they play a particularly important role in regulation between metabolic homeostasis and reproduction.
POMC/CART neurons secrete alpha-melanocortin-stimulating hormone (α-MSH), which acts as an agonist at melanocortin-3 and -4 receptors (MC3-R and MC4-R). AgRP / NPY neurons secrete agouti-related protein (AgRP) and neuropeptide Y (NPY), antagonists for MC3R and MC4R (16-18). α-MSH, produced by POMC/CART neurons, exerts a strong anorexigenic effect, and thus reduces food intake and induces weight loss, while AgRP and NPY show the opposite (orexigenic) effect, increasing food intake (19-21).
Both groups of neurons (POMC/CART and AgRP/NPY) express leptin and insulin receptors and the growth hormone secretagogue receptor (GHSR1). Both leptin and insulin stimulate POMC/CART neurons and inhibit AgRP/NPY neurons. Ghrelin, on the other hand, activates AgRP/NPY neurons, which then inhibit POMC/CART neurons by secreting GABA (22-26).
Many adipokines and neurohormones work together to form a network of complex regulations at hypothalamic level. As previously pointed out, the neurons of the ARC express adipokine receptors, such as receptors for leptin, ghrelin and insulin. It is therefore conceivable that kisspeptin participates in and is perfectly integrated in such a complex modulatory system regulated by metabolic signals. In fact, in experimental animals, it has been clearly shown that intermittent fasting conveys nutritional signals to the ARC in the hypothalamus. NPY and kisspeptin signals seem to be the main effectors of this, with changes in their expression disrupting the function of the hypothalamic-pituitary-gonadal axis in response to negative energy balance (27).
Moreover, recent studies indicate that many other hormones, such as orexin, spexin, waspin and nesfatin-1, may play an important role in the interaction between the reproductive system and metabolism (28-31).
Leptin
Leptin, an anorexigenic adipokine discovered in 1994 by Jeffrey Friedman, is a hormone secreted by adipocytes in white adipose tissue and it plays a key role in metabolic homeostasis in mammals (15, 32).
Leptin reduces appetite by decreasing the secretion of appetite stimulants such as NPY and AgRP. A decrease in leptin synthesis or insensitivity of leptin receptors may lead to weight gain and obesity. Leptin, acting through the receptors on POMC/CART and AgRP/NPY neurons, stimulates secretion of POMC and inhibits secretion of NPY in the CNS, and is thus responsible for transmitting information about energy reserves to the ARC (33).
In situations of energy imbalance, low levels of leptin cause a clear reduction in levels of Kiss1 transcripts in the hypothalamus. On the other hand, exogenous, systemic administration of leptin concentrations greatly increases expression of Kiss1 transcript levels (33). Similarly, ablation of leptin in ob/ob mice and hypoleptinemia in experimental diabetic rats diminish Kiss1 mRNA expression, while leptin infusion in both ob/ob mice and the rat model augments Kiss1 transcript levels (34).
Ghrelin
Ghrelin, discovered in 1999 in rat stomach cells, is produced in parietal cells of the stomach and fundus (X/A-like cells) and is considered to stimulate food intake in rodents and humans (orexigenic activity); it is secreted during fasting (35-38).
Acting through the GHSR1 receptor located on the neurons of the hypothalamus, it is involved in the central control of energy balance by stimulating appetite and increasing food intake. It is also responsible for preserving the accumulated adipose tissue (39).
Ghrelin increases food intake by activating AgRP/NPY neurons. At the same time, the AgRP/NPY neurons, stimulated by ghrelin, secrete GABA, which, by inhibiting POMC/CART neurons, enhances the orexigenic effects exerted in the hypothalamus (37). Ghrelin, by inhibiting the pulsatile release of GnRH, serves as a signal to suppress reproduction. Elevated ghrelin levels can delay sexual maturation and induce hypogonadotropic hypogonadism, and can lead to infertility (40, 41).
Ghrelin is suspected of affecting reproductive capacity by negatively impacting hypothalamic KISS1 and/or KISS1R mRNA expression. Moreover, as fasting raises plasma ghrelin concentrations, Ghrelin induces a decline in hypothalamic KISS1 gene expression and suppresses LH pulses (42).
Kisspeptin and pubertal development
Puberty is a unique process in human life. The term refers to the changes, determined by hormonal background, which enable an individual to be sexually active and able to reproduce (43).
Puberty, and particularly timing of puberty, is very complex and depends on many neuronal and neuroendocrine actions and relationships at the level of the hypothalamus. The period of puberty is associated with quiescence of GnRH pulsatile activity. The female reproductive cycle is regulated by proper pulsatile GnRH secretion (characterized by physiological amplitude and frequency of pulsatility). This proper normal pattern of GnRH secretion determines normal levels of gonadotropins — FSH (follicle-stimulating hormone) and LH —, which in turn are responsible for normal steroidogenesis at the level of the ovary (44).
The appearance of increased pulsatile release of GnRH plays a fundamental role in the process of puberty. GnRH is normally regulated by many excitatory and inhibitory factors. The main excitatory factors include kisspeptin and glutamate. Inhibitory factors are GABAergic neurons and opioidergic neurons.
The process of GnRH activation, which enables puberty onset, is not fully understood. Numerous neuropeptides and neurotransmitters such as neuropeptide Y, leptin, glutamate, neurokinin B, and mechanisms involved in the regulation of glial cells play a role in this process (45).
However, in this context, kisspeptin seems to play the most important and crucial role: the importance of this role is reflected in the phrase ”puberty begins with kiss” (46).
The significance of kisspeptin in puberty was first proved in animal studies. Studies on Kiss1R knockout mice showed that they do not reach puberty and are characterized by deficits in reproductive function in adulthood, low serum levels of gonadotropins and sex steroids, decreased gonad size, and impaired spermatogenesis and ovulation (47). In addition, in experimental animals it has been demonstrated that estradiol levels induce positive feedback on the induction of synthesis of local neurosteroids such as neuroprogesterone (neuroP) in hypothalamic astrocytes. NeuroP acts on kisspeptin neurons that express progesterone receptors so as to increase the kisspeptin expression and release necessary to trigger GnRH release and LH secretion (48).
Administration of kisspeptin to prepubertal female goats caused increases in serum gonadotropin levels and ovulation occurrence (49). Initial evidence from 2003 showed that inactivating mutations of GPR54 were related to absence of puberty and hypogonadism of central origin (5, 6). Later, different reports were published concerning mutations at different sites of the KISS1R gene (50, 51). Described patients were characterized by absence of or delay in pubertal development (52).
In 2008, an activating mutation of KISS1R was identified in a girl with central precocious puberty, underlining the importance of the role of kisspeptin in the physiology and pathology of puberty (53).
Serum kisspeptin levels have been found to be higher in children than in adults, and an increase in serum kisspeptin was observed at the moment of puberty both in girls and boys (54).
As mentioned, the kisspeptin is co-expressed with NKB and DYN, which is why we talk of KNDy neurons. NKB and DYN, together with kisspeptin, can be regarded as modulators of puberty onset. NKB acts as a positive regulator of kisspeptin secretion from KNDy neurons, while DYN exerts an inhibitory impact on kisspeptin release from these neurons (Fig. 1) (55).
Puberty onset is associated with metabolic status. The relationship between two hormones, kisspeptin and leptin, seems to play an important role.
During the prepubertal period, the amount of fat tissue increases. Fat tissue is source of leptin, and close to the time of puberty the secretion of leptin is augmented, leading to the observation of higher serum leptin levels. This leptin surge can be responsible for kisspeptin release from kisspeptinergic neurons (56). However, a key role for insulin cannot be excluded since pubertal maturation coincides with a physiological increase in the insulin resistance that plays a specific role in body mass increase, as well as in the development of all the organs (57). The role of kisspeptin in the process of puberty and pubertal disorders is very complex and broad and requires further investigation.
Temporal coupling between kisspeptin and gonadotropin pulsatile releases
A clear link between the stimulatory function of kisspeptin on gonadotropin secretion, through a specific direct action on the hypothalamic GnRH-secreting neurons, was demonstrated by several animal studies. Han et al. (58) and Zhang et al. (59) reported that kisspeptin was able to increase the firing rate of GnRH neurons in vitro, and Thompson et al. (60) demonstrated that kisspeptin was able to induce GnRH release from hypothalamic explants. Finally, intracerebroventricular injection of kisspeptin was reported to determine an incredible increase of GnRH in the cerebrospinal fluid of sheep and simultaneously the elevation of both gonadotropins in the blood circulation (61).
Although it has been supposed that kisspeptin might have a direct action on gonadotropic cells in the pituitary (8), it is very clear that kisspeptin acts on hypothalamic neurons and enhances GnRH secretion in such a way as to induce secretion of both gonadotropins from the pituitary. In fact, specific experimental designs in humans demonstrated that intravenous injection of kisspeptin-10 both in men (62) and subcutaneous injection in women (63) were able to increase LH pulse frequency and amplitude.
It is fundamental to note that while kisspeptin stimulates LH secretion in men, the effects of kisspeptin on LH release are variable in women, depending on the phase of the menstrual cycle, as well as on the different isoforms of kisspeptin, i.e., kisspeptin-10 and kisspeptin-54 (64). Although the physiological patterns observed in animal models cannot be considered identical to those seen in humans, they represent a putative hypothetical model of function. In rodents, the kisspeptin-producing neurons are found in the AVPV and in the ARC (65). Both neuronal populations are important in the control of GnRH secretion since the positive and the negative feedback signals to GnRH neurons target these distinct populations. AVPV neurons are critical for the GnRH-LH surge and ovulation in females and ARC neurons are involved in the tonic and pulsatile regulation of GnRH secretion in both sexes (65). In this animal model, AVPV kisspeptin-secreting neurons are sensitive to positive feedback (i.e., sensitive to low estrogen concentrations), while ARC kisspeptin neurons are sensitive to negative feedback (i.e., sensitive to high estrogen concentrations), thus regulating GnRH secretion (65, 66) (Fig. 1).
It is this kind of specific differential activity that underlies the adequate control of GnRH secretion and consequently of gonadotropin release from the pituitary thus regulating ovulation.
On the basis of what has been described thus far, kisspeptin is a key modulator of GnRH and thus of both gonadotropins. But is there any clear temporal relationship between kisspeptin and gonadotropin secretion that might be disclosed in humans? And, if there is, does it differ between different pathophysiological conditions?
Chronobiological studies in humans
It is only recently that the temporal relationship between kisspeptin and gonadotropins has been the focus of specific evaluation, to finally demonstrate the physiological coupling between these hormones mediated by GnRH.
In chronobiological evaluation, it is necessary to deal with two specific putative critical points: sample collection (i.e., sampling frequency) and hormone half-life (67). Since FSH has quite a long half-life, all studies to date have dealt with LH, whose half-life, being shorter than that of FSH (68), allows, through sampling every 10 minutes for at least 120 minutes, relatively simple identification of LH secretory episodes in the pulsatile profiles (67).
Experimental animal models have clearly demonstrated that kisspeptin and LH pulsatile release are closely linked by the simultaneous GnRH pulsatile secretion from the hypothalamus, but only recently have some studies been performed in humans to evaluate the endogenous and spontaneous secretory pattern of kisspeptin and its temporal coupling with LH.
As with previous studies on the topic of temporal coupling between hormones (69-72), studies on kisspeptin and LH were performed first to evaluate the presence of any pulsatile release and later to assess the possible presence of a temporal relationship between the two hormones. In this context, to avoid any inconvenience related to the different intrinsic secretory dynamics that each hormone has, only the start of the pulse and not its peak was considered in order to assess temporal coupling (73).
The temporal coupling evaluation was based on the determination of the specific concordance (SC) index between the secretory events of two distinct hormones (73). The SC index is a statistically based index that is computed taking in account the possibility that one or more of the secretory events of kisspeptin and LH might be occurring at exactly the same time by pure random chance. Due to this possibility, “Monte Carlo simulations were […] performed to study the frequency distribution of the SC index under the null hypothesis of random concordance, as previously described. For each of the 2 pairs of clinical data for each subject, 500 simulated pairs of simulated series were generated and frequency distributions of SC obtained. An SC value above the 95% percentile of the frequency distribution generated for that individual or group of subjects resulted in rejection of the null hypothesis at the p<.05 confidence level” (73).
Using this approach, Meczekalski et al. (74) demonstrated the presence of kisspeptin spontaneous pulsatile release in healthy women during the follicular phase of the menstrual cycle. In addition, this group was also able to show the presence of temporal coupling of kisspeptin secretory events with those of LH (74). This temporal coupling occurred at exactly time “0”, that is with no time lag: in fact, LH pulses occur exactly at the same moment in time as kisspeptin pulses. This evidence was rather important since it clearly suggests, through a simple experimental design in healthy women, the specific nature of kisspeptin as the main driver of LH secretion modulating hypothalamic GnRH discharge.
From this moment on, it seemed natural to suppose that kisspeptin might be involved, at least in part, in most, if not all, of the impairments that determine oligomenorrhea or amenorrhea and/or anovulation in women, and thus to investigate putative abnormalities of kisspeptin and of LH pulsatile release in classic pathophysiological states such as polycystic ovary syndrome (PCOS) and functional hypothalamic amenorrhea (FHA).
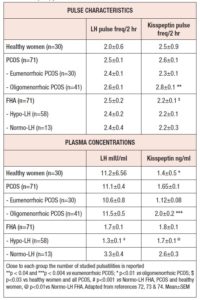
In an investigation of a rather large population of patients with PCOS, clear pulsatile release of kisspeptin was demonstrated (75), but surprisingly no temporal coupling with LH.
Given that this study was conducted to evaluate any possible differential aspect of kisspeptin regulation in PCOS subjects, the authors subdivided the PCOS population according to the presence of eumenorrhea or oligomenorrhea (intermenstrual interval > 45 days). After this subdivision, a completely different scenario emerged. In fact, the PCOS patients with normal menstrual cyclicity were found to have not only a kisspeptin pulse frequency and amplitude similar to healthy subjects (74), but also the same temporal coupling between LH and kisspeptin at time “0” described in healthy subjects. On the contrary, no temporal coupling was observed in PCOS patients with oligomenorrhea who, in addition, showed higher kisspeptin plasma levels and a higher pulse frequency than both eumenorrheic PCOS (75) and healthy women (74) (Table 1). These results clearly indicate that probably most, though not all, of the reproductive abnormalities observed in PCOS might be related to impaired kisspeptin secretion and/or modulation of GnRH hypothalamic discharge.
A further indirect demonstration of the pivotal role of kisspeptin in driving the ovarian cycle through GnRH-induced LH secretion was provided by an investigation of a large group of subjects affected by FHA. In fact, the analysis of kisspeptin and LH pulsatile profiles disclosed a distinct spontaneous pulsatile secretion of kisspeptin as well as its temporal coupling with LH (76). Although kisspeptin pulse frequency was found to be in the same range found in both healthy women and women with PCOS (Table 1), its plasma concentration was lower. In addition, when women with FHA were considered according to their LH plasma levels, hypo-LH FHA patients showed lower kisspeptin and LH plasma concentrations than healthy and PCOS subjects, even though the pulse frequency of both hormones was similar.
This observation clearly supports the concept that in FHA patients the neuroendocrine mechanisms regulating reproduction through kisspeptin and affected by stressor(s) are able to change kisspeptin pulse amplitude rather than pulse frequency, thus also reducing LH secretion.
Recently Podfigurna et al. (77) reported that in patients with FHA, CRH plasma concentrations are inversely correlated with kisspeptin, FSH and LH concentrations, and thus demonstrated a specific correlation between stress-induced CRH secretion and the impairment of GnRH hypothalamic release in FHA.
Concluding remarks
In the light of what is discussed herein, kisspeptin is absolutely the driver and the main promoter of LH secretion, and drives reproduction through stimulation of GnRH neurons.
Peculiar pathophysiological conditions seem to affect the ability to ovulate, but in most cases the temporal coupling between kisspeptin and LH secretory episodes is preserved. It is interesting to note that this coupling is present in FHA patients and in eumenorrheic PCOS subjects with normal or low hormonal/metabolic indexes, such as leptin and insulin.
In fact, this coupling between kisspeptin and LH disappears in oligomenorrheic PCOS patients, who can be supposed to have higher levels of both these hormones as well as of other peptides (i.e., adiponectins) that are well known to modulate the neuroendocrine control of reproduction. It can be presumed that direct and/or indirect metabolic signals, such as insulin, might be responsible for this interference, especially in PCOS patients who are often characterized by compensatory hyperinsulinemia and overweight/obesity.
We might reflect on the following facts: metabolism has been demonstrated to be the main driver of reproduction in slim/thin/anorectic subjects. Lack of energy has long been (i.e., from prehistoric Paleolithic times and before) the main signal that might drive the ability to conceive, in other words, leading pregnancy to be avoided when food was scarce. Lack of food and energy leads to insulin and leptin reduction together with kisspeptin and an increase in cortisol, all specific effectors of reduced reproductive ability. Human evolution, over thousands of years, brought a progressive improvement in feeding and food sourcing that permitted better stability and control of survival, and therefore higher levels of pregnancy. It is intuitive that over hundreds of thousands of years, and up to relatively recently, overweight or obesity were not contemplated by human evolution and biology. In fact, both factors began to appear only in the last couple of millennia, thanks to improved human skills that permit a better food collection and its storage.
A remarkable consideration is the fact that excess energy storage impairs reproduction through the same players that mediate this same effect when energy is lacking, namely leptin, insulin, adiponectins and others. These act by modulating and promoting a change in the stability of kisspeptin control of GnRH-induced LH secretion, and disrupting ovarian function through abnormal coupling, similarly to what is observed in oligomenorrheic PCOS patients. On this basis, and in view of these considerations, it is worth noting that kisspeptin administration might be considered a putative therapeutic strategy in specific clinical conditions (78).
Ludwig Feuerbach (1804-1872), a great philosopher, dozens of years before the evolution of the modern concept of scientific research, stated in a book published in 1862 that “mankind is what it eats”. Absolutely!
Conflict of interest
The authors declare no conflict of interest.