Introduction
Cryopreservation of human embryos was first described in 1983 [1]. Since then, the proportion of frozen embryo transfers (FET) has been increasing in the field of assisted reproduction technology (ART) [2]. This rise in the number of FETs can be ascribed to refinement in embryo vitrification methods resulting in improved post-thaw embryo survival rates with an increase in live birth rates [3,4]. FETs are excellent alternatives for patients unable to complete fresh transfers, owing to an inadequate endometrial lining, inappropriate hormone levels, desire to pursue preimplantation genetic testing, and for those at risk of developing ovarian hyperstimulation syndrome [5]. Several studies have reported that FET achieved higher pregnancy rates and lower complications rates compared with fresh embryo transfers [6-8]. The perceived benefits of FET have already led to its widespread adoption into clinical practice.
In FET cycles, different regimens are used to prepare the endometrium including natural cycles (NC), ovulation induction cycles (OI) and hormone replacement cycles (HRT), synchronizing the timing of endometrial growth with embryonic development. Currently, there is no data to support one method of endometrial preparation over another [9-11]. Moreover, very few studies have addressed the issues of cost-effectiveness, and convenience of FET protocols [11]. With the increasing utilization of FET, there remains a need to analyze these factors and address these key questions. Accordingly, we performed a propensity score matched retrospective analysis comparing cost-effectiveness, and cycle outcomes of three commonly used endometrial preparation protocols.
Materials and methods
Study design and patient population
This was a single-centre, retrospective cohort study. We searched the institutional review board approved database of patients who underwent thawed blastocyst transfers, between January 2009 and June 2015 at our centre. As a routine, patients undergoing IVF treatment in our centre provide informed consent to the anonymous use of their data for retrospective reviews.
The embryos were derived from either IVF or IVF using intracytoplasmic sperm injection treatment cycles. We excluded cycles that used donor oocytes and day 3 cleavage-stage embryo transfers. All eligible FET cycles were categorized into three groups based on the protocol used for endometrial preparation. The decision regarding the protocol was made based on the experience of the physician and patient characteristics. Natural cycle protocol was the preferred choice for women with ovulatory cycles, while ovulation induction and hormone replacement cycles were recommended for those with a history of oligo-ovulation or anovulation.
Study procedures
Ovulation induction cycle with letrozole (OI - Group-1): Patients were administered 5 mg per day letrozole on day 3 of the menstrual cycle for five consecutive days, after a baseline ultrasound. Follicle development was monitored using serial transvaginal ultrasounds (TVUS) and serum estradiol levels (E2) from day 10 of the menstrual cycle. If follicle diameters were 18-25 mm and the endometrial thickness 8 mm-10 mm, patients were given instructions to administer 250 micrograms recombinant human chorionic gonadotropin (rhCG) subcutaneously to trigger ovulation and 36-48 hours after that intramuscular progesterone in oil (IM P 50 mg) be administered for 5 days and thawed embryos be transferred. If there was no follicle with diameter ≥14 mm after 10–14 days or endometrial thickness (ET) did not reach 8 mm, the cycle was cancelled.
Natural cycle (NC – Group-2): Women were instructed to start point of care testing daily with ovulation prediction kits from the 10th day of the menstrual cycle to assess for ovulation and to notify the clinic when the result was positive. TVUS and serum hormone (LH, P) testing to confirm ovulation was done two days after a positive ovulation surge. IM P 50 mg was administered for 5 days and thawed embryos were transferred 6 days after the LH-surge. If the pregnancy was successful, the patient continued on P supplementation until 10–12 weeks of gestation. If there was no follicle with a diameter ≥14 mm after 10–14 days or ET did not reach 8 mm, the cycle was cancelled.
Hormone replacement cycles after GnRH down-regulation (HRT - Group-3): Patients were given subcutaneous (SC) injections of leuprolide acetate, 0.5 mg/day (after 2 weeks of oral contraceptives (OCP) with a 5-day overlap of the GnRH agonist and OCP, starting from the mid-luteal phase of the cycle preceding the actual FET cycle. After confirming appropriate down-regulation (E2 levels <150 pmol/L, TVUS showing quiescent ovaries and ET less than 5 mm), patients were started on oral micronized 17β-estradiol at a dose of 2 mg daily and titrated to 6 mg daily over 12 days. After 12-14 days, TVUS measurement of ET and serum E2 levels were performed. When ET was ≥ 8 mm, GnRH agonist treatment was discontinued, IM P 50 mg was administered for 5 days and thawed embryos were transferred. Both estradiol and P were continued until 10-12 weeks of gestation if the pregnancy test was positive. If ET was considered inadequate, the estrogen dose was increased to 8 mg/day and TVUS was repeated after a week. If ET remained <8 mm, the cycle was cancelled.
Vitrification technique was used for cryopreserving the embryos. Blastocysts were graded according to the Gardner criteria. The number of embryos to be transferred was determined per American Society of Reproductive Medicine (ASRM) guidelines. Blastocysts were thawed on the day of embryo transfer approximately 1–3 hours before transfer. A transabdominal ultrasound-guided embryo transfer was performed. Of note, the embryo freezing-thawing protocols, embryo transfer catheter, and operator skills were the same for all the patients.
Pregnancy test (serum βHCG) was done after 12 days of embryo transfer and a TVUS was scheduled 4–5 weeks after a positive pregnancy test to determine the number, location of gestation sac and viability of the embryo.
Cycle outcomes
The primary outcome measures of our study were cost per cycle, cost per pregnancy and cost per live birth. Other secondary outcomes of interest were implantation rate, clinical pregnancy rate, live birth rate, spontaneous abortion rates. ART outcomes were defined per the International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology [12]. Live birth data were collected by contacting patients and the live birth rate was defined as the number of live births divided by the number of FET cycles. Clinical pregnancy was defined as pregnancy documented by ultrasound that shows a gestational sac in the uterus. The implantation rate was defined as the number of maximum fetal hearts detected by ultrasound, out of the total number of transferred embryos. Spontaneous abortion was defined as a pregnancy ending in the spontaneous loss of the embryo or fetus before 20 weeks of gestation.
Cost-effectiveness analysis
We calculated the total cost to complete a treatment cycle in propensity score-matched FET cycles. The cost of the preceding IVF/ICSI cycle was incurred by all women before starting the FET cycle and so it was not included in the analysis. We reviewed the mean number of office visits, costs involved in monitoring the cycle progression (ultrasound, lab tests including hormone assays), costs of medications, embryo transfer and IVF lab charges. Costs were calculated by multiplying units used with unit prices. Unit prices were obtained from the financial department of our centre. Based on cycle outcomes, cost per pregnancy and cost per live birth were calculated and compared. All of the prices were expressed in American dollars (USD).
Statistical analysis
We implemented a propensity score matching approach to decrease bias when comparing outcome variables, due to an imbalance in covariates and to simulate a randomized clinical trial (RCT) [13]. A propensity score was estimated from a logistic regression model that considered multiple baseline covariates (age, body mass index [BMI], cause of infertility, number of previous IVF and FET cycles). Matching of cycles in each group was performed on the closest score (nearest neighbor matching) without replacement model [14]. Cycles were matched initially between OI-Group-1 and NC Group-2. Then the selected cycles were matched with three controls in the HRT group. We performed outcome analysis in this matched group. Data are presented as mean ± standard deviations (SD) for continuous variables, or as a percentage for categorical variables. A comparison of the mean values was performed using one-way analysis of variance (ANOVA) for independent samples. For a comparison of the categorical data, the Chi-square test was performed. Two-tailed P value was calculated and considered significant if < 0.05. If P < 0.05, Tukey HSD (honest significant difference) post-hoc test was used for pairwise comparisons. Propensity score matching and statistical analyses were performed using R statistical software environment version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and Statistical Package for Social Sciences (SPSS) version 23.0.
Results
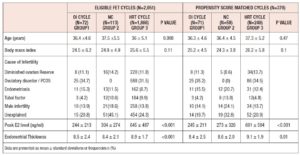
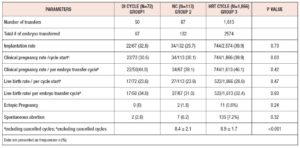
The initial analysis included 2,051 FET cycles; 72 cycles used OI with letrozole (Group-1), 113 were NC (Group-2) and 1,866 cycles used HRT (Group-3). The baseline characteristics of the study population are shown in Table I. Women in Group-1 tended to be older (62% were aged more than 35 years). After propensity score matching, the type of infertility (P=1), number of FET cycles (P=0.49), the grade and mean number of blastocysts transferred (P=0.33) were similar between three groups. Cycle outcomes are shown in Table II. Clinical pregnancy rate per cycle start was higher in Group-3 (Group- 1: 30.6% (22/72), Group-2: 30.1% (34/113) and Group-3: 39.9% (744/1,866), P=0.03) but clinical pregnancy rate per embryo transfer were similar in all three groups (Group-1: 44% (22/50), Group-2: 39.1% (34/87) and Group-3: 46.1% (744/1613), P=0.42). There were no statistically significant differences in implantation rate, spontaneous abortion, ectopic pregnancy rates and live birth rates between the three groups.
Propensity score matched analysis
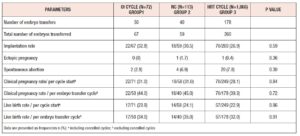
After propensity score matching, there were 71 cycles in Group-1, 58 in Group-2, and 249 in Group-3 (total of 378 cycles). The baseline characteristics of matched groups (Table I) were much more comparable. Peak E2 levels were lower in Group-1 (245 ± 213 ng/ml, P <0.001) and ET was highest in Group-3 (9.1 ± 1.9 mm, P=0.01). After matching, no statistically significant difference was detected in cycle outcomes between the three groups (Table III). The overall cycle cancellation rate for any reason was 29%, this included cycles that were cancelled due to inadequate endometrial and ovarian response and patient-initiated cancellations. There was no statistically significant difference in the number of cancelled cycles between the matched groups, Group-1: 29.6% (21/71), Group-2: 31% (18/58) and Group-3: 28.5% (71/249), P= 0.36).
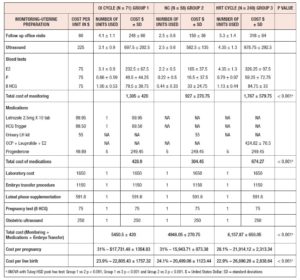
Table IV presents the calculated cost per cycle along with cost per unit and number of units used. Group-1 and Group-2 had fewer follow-up visits than Group-3 (4.1 ± 1.1 vs 2.5 ± 0.6 vs 5.3 ± 1.4 respectively). This was reflected in the cost of monitoring, with Groups 1 and 2 having lower costs in comparison to Group-3. The cost of medications was also lower in Groups 1 and 2 as compared to Group-3. The costs of luteal-phase supplementation, IVF laboratory costs and embryo transfer procedure costs were similar between groups. The total cost per cycle was statistically higher for Group-3 (Group-1: $5,450.5 ± 420.00 vs Group-2: $4,948.05 ± 270.75 vs Group-3: $6,157.87 ± 650.05, P < 0.001). We observed a statistically significant lower cost per pregnancy in Group-1 and Group-2 in comparison to Group-3 ($17,731.48 ± 1,354.83 vs $15,943.71 ± 873.38 vs $21,914.12 ± 2,313.34, respectively, P < 0.001). The cost per live birth was statistically higher after HRT cycles (Group-3: $26,890.26 ± 2,838.64 vs Group-2: $20,499.06 ± 1,123.44 vs Group-1: $22,805.43 ± 1,757.32, P< 0.001).
Discussion
Our results showed that natural and letrozole OI cycles are cost-effective alternatives to HRT cycles for endometrial preparation in FET. The mean treatment cost to achieve a live birth was higher after HRT cycles compared to NC and letrozole OI cycles. The cost difference was mostly explained by higher costs of monitoring (difference of $840 ± 309 (HRT vs NC) and $462 ± 159.75 (HRT vs OI cycle) and medications per treatment cycle (difference of $369.82 ± 70.3 (HRT vs NC) and $245.37 ± 70.3 (HRT vs OI cycle)) for HRT cycles. The pregnancy and live birth rates were comparable between the three groups.
To the best of our knowledge, ours is one of the first studies to report on the cost-effectiveness of FET protocols. A non-inferiority RCT compared modified NC (mNC) with HRT cycle and reported comparable live birth rates and costs for each of the endometrial preparation methods [11]. Their protocol had more frequent ultrasound monitoring visits that possibly increased the cost of mNC. Other than treatment-related cost, indirect costs like travel expenses were also included in their analysis. Our study suggests that direct costs per cycle is lower with natural and letrozole OI cycles than down-regulated HRT cycles.
Upon analyzing the eligible study population, we found a higher clinical pregnancy rate with HRT cycles. This group had relatively younger women with lower BMI. After propensity score matching of baseline characteristics, the clinical pregnancy and live birth rates were similar between the three groups. Xiao et al. [15] and Morozov et al. [16] reported higher pregnancy rates in NC compared with HRT cycles. Nonetheless, both used non-blastocyst embryos and evaluated pregnancy rates, which is less clinically meaningful as compared with live birth rates. Zheng et al. [17] noted higher pregnancy rates in HRT cycles. But, the study used non-blastocyst embryos and relied on high thresholds of progesterone to time ovulation in the NC regimen. A recent RCT reported a non-significant higher pregnancy rate with letrozole compared to the HRT group [18]. Our findings are in agreement with the results reported by a Cochrane review in 2017 and a recent meta-analysis which concluded that no FET regimen was superior to another in terms of clinical pregnancy rates or live birth rates [9-11].
The endometrial preparation protocols described in our study are routinely used in clinical practice. We opted letrozole for the OI FET protocol because it induces single follicle development, unlike gonadotropins. It exhibits no anti-estrogenic effects on the endometrium and cervical mucus, unlike clomiphene. It also improves endometrial receptivity by increasing αVβ3 integrin and pinopode expression on the endometrium during implantation [19,20].
In our study, peak E2 levels in letrozole OI cycles were significantly lower than in NC and the HRT cycles, whereas ET seems to be similar to that of NC. These findings concurred with other studies that used the letrozole OI protocol in FET cycles [18,21,22]. Letrozole is a direct aromatase antagonist and inhibits the conversion of androgen into E2. This enables lower peak E2 levels at follicle maturation, decreasing ubiquitination of the estrogen receptor α. This causes up-regulation of estrogen receptors, increasing its sensitivity to subsequent estrogen rise [23]. Therefore, faster proliferation of the endometrial epithelium and stroma with improvement in blood flow to the uterus and endometrium occurs, which facilitates implantation [24]. Ma et al. [25] have demonstrated that the time required for appropriate uterine receptivity (‘‘nidation window’’) was inversely correlated with the serum E2 levels.
Given the lack of difference in reproductive outcomes between the studied FET protocols, the decision to choose a treatment method may be based upon other factors. Logistical aspects, such as the number of monitoring visits, cost of medication and overall cost-effectiveness of the protocol are all important factors to incorporate into the shared decision-making process with the patient. In NCs the main advantage is the avoidance of exogenous hormone supplementation which reduces the treatment cost. Patients can conveniently perform home urine testing to determine the onset of the LH surge. False-positive or negative testing, due to substantial inter-patient and cycle variation in LH amplitude and shape, can lead to default planning of thawing and transferring of embryos [26]. This must be communicated to patients during counseling. Considering OI cycles, letrozole is economical and easy to administer. Letrozole stimulation results in a hormonal profile and endometrial histology similar to that of spontaneous cycles [27]. HRT cycles may confer the advantage of greater control over the cycle and transfer date. But this technique seems to be relatively expensive. In our study, the number of office visits for monitoring the treatment was relatively lower for NC and letrozole OI cycles. Also, the cost per cycle and live birth were significantly lower for these two groups.
The strength of our study is that we analyzed the outcomes at the cycle level, not on the patient level. This mimics real-life clinical practice. In agreement with Alur-Gupta et al. [28] we followed patients to live birth, to evaluate whether the effects of differences in endometrial preparation type persisted beyond the first trimester. This is because of increasing evidence indicating that the peri-implantation environment may influence perinatal outcomes [29]. Furthermore, it is one of the few studies that addressed cost-efficiency. There are some limitations to our study that should be considered when interpreting the findings. First, given our retrospective study design, we could not completely avoid selection biases and other inherent biases therein. The patients were assigned to groups based on clinical practice. There was an unequal distribution of patients among the groups, particularly the small number of samples in the OI group. To decrease biases to the maximum extent, we used propensity score matching to balance the confounding factors and provide more credible results. However, propensity score matching techniques can balance observed variables but cannot control for unmeasured variables. To overcome this limitation, a RCT would be ideal. Next, we also did not perform power analysis in the current study. Also, in our analysis we have considered only direct health care costs and did not include indirect costs such as the value of lost productivity due to time off from work, travel expenses or the costs of wasted attendances arising from subsequent cancellation in the cost calculation.
In conclusion, the present study suggests that NC and letrozole OI cycles are cost-effective alternatives to HRT cycles for endometrial preparation in FET with no significant difference in live birth rates. There are other commonly employed FET protocols like estradiol hemihydrate followed by intravaginal progesterone for endometrial preparation. It would be interesting to evaluate the cost effectiveness of other protocols as well in future studies. We believe the results of our study will provide important information which could help clinicians counsel women undergoing a FET. Large prospective RCTs are needed to verify whether these methods are equivalent, and to confirm our findings on treatment costs.
Conflict of Interest Statement: The authors declare having no conflicts of interest.