Introduction
Acne vulgaris, or acne, is a common skin condition that affects 9.4% of the world population [1]. More than 85% of adolescents suffer from acne, which is moderate to severe in about 15–20% of patients [2]. Acne often persists into adulthood. 64% of individuals in their 20s and 43% of individuals in their 30s show signs of visible acne. A study of more than 2000 adults demonstrated that 3% of men and 5% of women still had definite mild acne at the age of 40 to 49 years [2].
Acne can be of non-inflammatory or inflammatory type. The first is characterized by open or closed comedones, which are small papules with or without a central black keratin plug, respectively [3]. The inflammatory type is categorized by papules, nodules and pustules. Both types may coexist [3] but inflammatory acne is the most common chronic inflammatory disease in adolescents and young adults [4].
Acne results in physical symptoms such as soreness, itching, and pain, but its main effects are caused by the visible lesions in the skin. Since this is an organ on social display [5], the psychological effects of acne can be devastating and may lead to low self-esteem, anxiety, suicidal ideation, shame, embarrassment and social inhibition [5].
Although the pathogenesis of acne is not fully understood, it is commonly accepted that it involves hair follicles in the skin associated with the pilosebaceous unit, an oil gland. The distribution of acne corresponds to the highest density of pilosebaceous units in the face, neck, upper chest, shoulders, and back [5]. Several factors are implicated in acne pathogenesis, such as (1) keratin plugs blocking sebum outflow to the skin surface, (2) hypertrophy of sebaceous glands during puberty under the influence of androgens, (3) Propionibacterium acnes converting sebum-containing lipids into proinflammatory fatty acids, and (4) secondary inflammation of the affected follicle [3].
Since sebaceous glands are androgen dependent, hormonal control reduces the sebum production that is initially induced by androgens. Available treatments therefore include hormonal control, but also antimicrobial therapies, vitamin A derivatives, such as 13-cis-retinoic acid (isotretionin), and physical therapies (electrocauterization, cryotherapy and comedone extraction). Currently, there is also a considerable choice of alternative and complementary medicines [6].
Combined oral contraceptives are indicated in female adolescents and women of childbearing age who suffer from moderate or severe acne [5]. Several studies have demonstrated the efficacy of dienogest/ethinylestradiol for the treatment of acne vulgaris [4,7,8]. Ethinylestradiol is a synthetic hormone derived from estradiol which reduces ovarian androgen production and stimulates liver production of sex hormone-binding globulin (SHBG). SHBG decreases biologically active free testosterone in the female body, ultimately reducing activation of the sebaceous glands. Dienogest is a fourth-generation progestin that blocks androgen receptors in target organs and reduces the activity of 5-reductase, which converts testosterone into 5-dihydrotestosterone [9]. Although all contraceptives can be used to treat hormone-related acne, progestins are usually preferred because they possess no androgenic activity [6].
Palacio-Cardona et al. reported that continued use of the 2 mg dienogest/0.02 mg ethinylestradiol combined oral contraceptive pill reduced inflammatory as well as non-inflammatory acne lesions in women aged between 18 and 30 years of age. It was also demonstrated that ethinylestradiol/dienogest diminished levels of hair and skin greasiness, and its overall effect was rated “good” or “very good” by 87.5% of surveyed women [8].
The present study was conducted to evaluate satisfaction, among Portuguese women, with the use of 2 mg dienogest/0.03 mg ethinylestradiol (Sibilla®, Gedeon Richter Plc., Budapest, Hungary) for the treatment of moderate acne. Secondary objectives included self-assessment of skin comfort and acne severity.
Methods
This was a non-interventional, prospective, multicentric study, conducted to assess women’s satisfaction with the use of the combined oral contraceptive containing 2 mg dienogest/0.03 mg ethinylestradiol for the treatment of moderate acne. The study was approved by the ethics committee and conducted in accordance with the Declaration of Helsinki.
Recruitment took place during regular gynecological appointments at Portuguese private clinics. 103 women were recruited, aged between 16 and 45 years, diagnosed with moderate acne, with indication for 2 mg dienogest/0.03 mg ethinylestradiol. The decision to prescribe the drug was clearly dissociated from the decision on whether or not to include the patient in the study, and the medicine was prescribed according to the approved therapeutic indications. Exclusion criteria included use of hormonal contraception in the last three months and women who were, at the time of recruitment, medicated with isotretinoin.
After checking fulfilment of all eligibility criteria, the women signed the informed consent form and answered a baseline (BS) questionnaire that requested information on their demographic, social and personal characteristics, as well as medical background and family history of acne. Women were followed for 13 menstrual cycles and at 3 (3M), 6 (6M) and 12 (12M) months into treatment answered another questionnaire, administered via e-mail or telephone. E-mail was the preferential contact route and only residual surveys were done via telephone. The study timeframe was from June 2018, when the first participant initiated the therapy, until March 2020, when the last participant completed 1 year of intake of 2 mg dienogest/0.03 mg ethinylestradiol. In all questionnaires, including the BS questionnaire, participants reported their skin type (oily, mixed, dry), acne lesion count, and acne severity based on self-evaluation diagrams, and also rated their skin comfort. Acne lesion count was based on a face division system proposed by Ramli et al. [10]. Women were asked to count the different lesions in the six described segments. In turn, acne severity was ranked using the scale of 10 images suggested by Patel et al. [11]. In addition, in the follow-up questionnaires, they were asked to rate their satisfaction with 2 mg dienogest/0.03 mg ethinylestradiol, and to indicate whether they intended to continue the treatment. Participants who withdrew prematurely from the study or who did not complete all the study procedures were not replaced.
Data analysis was performed with SPSS Statistics for Windows, IBM Corp., Version 21.0. (Armonk, NY), using the Fisher’s exact test, Wilcoxon test, Friedman test and one-way ANOVA. Information about the different statistical analyses is included in the respective figure legends, table footnotes and in the following text.
Results
Demographics and BS characteristics
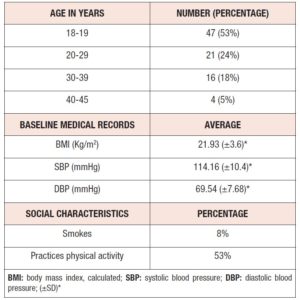
A total of 103 women were recruited, of whom 11 were found to be non-eligible. Participants initiated the treatment at the dosage of 2 mg dienogest/0.03 mg ethinylestradiol daily and were followed up over 13 menstrual cycles, answering questionnaires at 3M, 6M and 12M into the treatment. A total of 38 participants dropped out of the study, specifically 4 at BS, 11 at 3M, 12 at 6M, and 11 at 12M. The majority of these women were lost to follow up. The others stopped taking the medication on their own initiative (14), due to medical indication (4), or due to incomplete symptom relief (2). In total, 88 questionnaires were obtained at BS, 77 at 3M, 65 at 6M, and 54 at the study endpoint. Table 1 summarizes information on the demographics, social and medical records of the women who answered at BS. 47 of the participants were aged 18 or 19 years and only four between 40 and 45 years. Mean body mass index (BMI) and systolic as well as diastolic blood pressure (SBP and DBP) values fell within the respective normal ranges. Only 8% of the participants were smokers and more than half (53%) practiced physical activity regularly.
Acne evolution
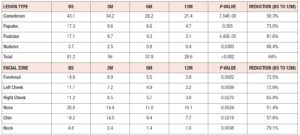
At all timepoints, participants self-assessed the number of acne lesions per type over the entire face, including comedones (closed and opened), papules, pustules and nodules. The face was defined as the area bounded by ears, hairline and lower mandibular margin. Areas 1 cm below the eyes and around the brows and lips were excluded from lesion counting. Lesion count was reported for each of the 6 locations (forehead, nose, right cheek, left cheek, chin and neck) according to Ramli et al. [10]. Average total lesion counts (table 2) decreased over time, from 81.2 lesions at BS to 29.6 lesions at 12M. This decrease represents an approximately 64% reduction, indicating that treatment with 2 mg dienogest/0.03 mg ethinylestradiol was effective in decreasing the average number of lesions. Average count per lesion type and per facial zone decreased at all timepoints (table 2), and differences between timepoints were statistically significant for all lesion types and for all facial zones. In detail, nodules and pustules showed the highest average reduction from BS to 12M, 88.4% and 81.6%, respectively, and comedones the lowest (50.3%). All 6 facial zones exhibited more than 50% average reduction from BS to 12M. The neck area revealed the highest average reduction (79.1%) and the nose the lowest (51.4%).
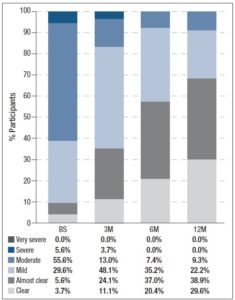
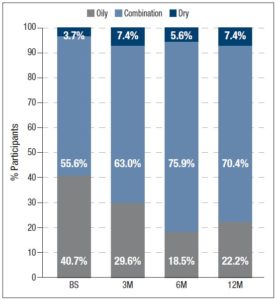
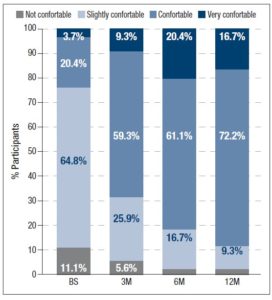
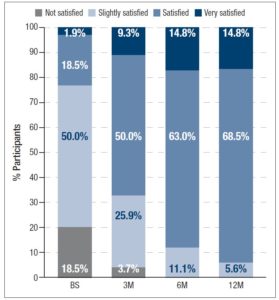
In addition, participants assessed their acne severity using a validated visual analog scale consisting of 10 drawings of faces. These images depict acne severity, ranked into 6 classes: “Clear”, “Almost Clear”, “Mild”, “Moderate”, “Severe” and “Very Severe” [11]. Statistically significant differences in acne severity were recorded over the different timepoints, excepting between 6M and 12M. (figure 1). At 12M, none of the women reported “Severe” or “Very Severe” acne and the percentage of women reporting “Clear” or “Almost Clear” skin has increased by 25.9% and 33.3% from BS, respectively. Treatment with 2 mg dienogest/0.03 mg ethinylestradiol also significantly altered the skin type (figure 2) Whereas 40.7% considered their skin oily at BS, only 22.2% did so after 12M of treatment. Participants were also asked to grade their perceived skin comfort at all timepoints (figure 3). After 12M of treatment with 2 mg dienogest/0.03 mg ethinylestradiol, only 1.9% of participants did not report comfortable skin, whereas 72.2% did, representing an increase of 51.9% compared with BS comfort levels. Likewise, satisfaction with skin (figure 4) increased throughout the 12M study, as demonstrated by the percentage of participants who declared themselves satisfied with their skin, which rose from 18.5% to 50.0% in the first 3 months. At the end of the treatment, 83.2% of individuals were “Satisfied” or “Very Satisfied” with their skin. In addition, there was a strong correlation between skin comfort and satisfaction with skin: r=0.7738 (3M), r=0.8688 (6M) and r=0.9185 (12M). These results indicate that satisfaction with skin largely depends on how comfortable it feels.
Participant satisfaction
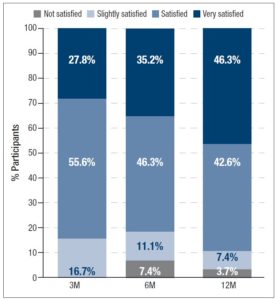
Satisfaction with 2 mg dienogest/0.03 mg ethinylestradiol treatment (figure 5) increased over time. At 3M, 83.4% of the women already felt “Satisfied” (55.6%) or “Very Satisfied” (27.8%) with 2 mg dienogest/0.03 mg ethinylestradiol for acne treatment. At the end of the study, 88.9% of the women were “Satisfied” (42.6%) or “Very Satisfied” (46.3%). Differences in satisfaction between 6M and 12M were statistically significant (p<0.017, Wilcoxon test), but not between 3M and the other timepoints.
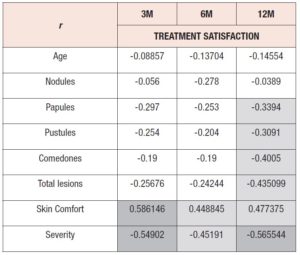
In order to understand how treatment satisfaction was correlated with other variables, Pearson correlation coefficients (r) were calculated between treatment satisfaction and (1) participant age, (2) lesion counts (total and per type), (3) acne severity and (4) skin comfort (table 3), for all timepoints. As expected, satisfaction with treatment was negatively correlated with average lesion count. Satisfaction and lesion type showed a moderately strong association at 12M for papules, pustules and comedones. Otherwise, weak associations were found. Severity was also negatively correlated with satisfaction at all timepoints, presenting the strongest association among all the variables analyzed (r=-0.566) at 12M. At all timepoints, skin comfort perception positively influenced treatment satisfaction, but the correlation was stronger at 3M (r=0.586) than at 12M (r=0.477). Participant age was poorly correlated with satisfaction.
Further, to understand whether satisfaction with the 2 mg dienogest/0.03 mg ethinylestradiol treatment depended on other skin interventions, we examined possible relationships between daily face treatments, esthetic treatments and other medical treatments, and satisfaction at 12M. Of the 88.9% who were “Satisfied” or “Very Satisfied” with 2 mg dienogest/0.03 mg ethinylestradiol, 63% used face care treatments, such as cleansing gel, cleansing mask or anti-acne cream on a daily basis. The majority of the participants did not perform esthetic treatments (81%). Prior to the study, 24% of the participants had used another anti-acne medical treatment. Satisfaction with 2 mg dienogest/0.03 mg ethinylestradiol and reported skin comfort were independent of all types of treatments (p>0.05, Fisher’s Exact test).
Intentions regarding future use of 2 mg dienogest/0.03 mg ethinylestradiol
Participants were asked whether they intended to continue with 2 mg dienogest/0.03 mg ethinylestradiol treatment in the long term. Just 3M after initiating the treatment, 92.6% of the women wished to continue with it in the long term. Interestingly, this intention was found to be dependent on the grade of acne severity. However, at 6M and 12M, the intention to continue treatment (87.04% and 85.2% respectively) was independent of the grade of acne severity. Differences, over time, in the percentages of women intending to continue the treatment were not statistically significant (p=0.197, Friedman test).
Discussion
Treatment with 2 mg dienogest/0.03 mg ethinylestradiol was effective in reducing all lesion types throughout the 12-month study. The average total lesion count fell by 63% and improvements in both non-inflammatory (comedones) and inflammatory (papules, pustules and nodules) acne were reported. These findings are similar to, although not as strong as, those reported by others [4]. In fact, Palacio-Cardona et al. reported a 94% reduction in acne lesions when using a combined oral contraceptive pill containing 0.02 mg of ethinylestradiol instead of 0.03 mg. The superior results of that study compared with this one might be explained by the fact that, in their study, the reported median value at BS for pustules and nodules was 0 and the oldest participant was 30 years old.
At 3M into treatment, treatment satisfaction showed the strongest association with skin comfort and acne severity. In turn, at 12M, total lesion count (r=-0.435) was also strongly associated with treatment satisfaction, as was comedone count (r=-0.4005). Thus, satisfaction showed a stronger association with the global facial appearance (severity) and how women feel about their skin (comfort) rather than with the number of facial lesions.
Complementary treatments [6], such as chemical peels [12] and light treatments [13], have been used for acne management. Interestingly, satisfaction was independent of whether esthetic treatment was performed during the course of the study. This fact suggests that regardless of whether or not participants performed additional treatments, the reported levels of satisfaction are primarily due to the effects of 2 mg dienogest/0.03 mg ethinylestradiol. Cleansing gels and moisturizers have also been recommended for the daily treatment of acne [14]. However, in this study, treatment satisfaction was independent of whether or not participants cared for their skin on a daily basis.
Intention to continue showed a decreasing trend, which was not statistically significant. Although severity emerged as the predominant parameter influencing satisfaction, intention to continue the treatment depended on it only at 3M, and not at the subsequent follow-up timepoints. This observation might be explained by other variables not assessed in the current study, such as decisions to suspend or change treatment, or not feeling comfortable with long-term use of hormonal treatments.
Conclusion
Continued use of the 2 mg dienogest/0.03 mg ethinylestradiol reduced inflammatory and non-inflammatory acne lesions in reproductive-age women between 18 and 45 years of age, previously diagnosed with moderate acne. Most of the women (88.9%) declared that they were at least “Satisfied” with 2 mg dienogest/0.03 mg ethinylestradiol treatment and expressed their intention to continue with it (85.2%) in the long term.
Acknowledgements
The authors would like to thank the medical doctors who recruited participants for the study. Data collection, statistical and editorial support were performed by an independent committee, funded by Gedeon Richter Plc.
Conflict of interest statement: No hospital, institution or medical doctor has received reimbursements for data collection. Statistical and editorial support was provided by an independent committee and was funded by Gedeon Richter Plc. Daniel Pereira da Silva is a Medical Advisor of Gedeon Richter Portugal. The remaining authors have no conflict of interest in relation to this article to disclose.