Introduction
The composition of the vaginal microbiome is dynamic and undergoes several changes corresponding with hormonal fluctuations throughout a woman’s reproductive life. The vagina harbors several microorganisms that exist in a regulated and mutualistic relationship within the microbiome (1). The composition of the bacterial species in the vaginal microbiome changes significantly in response to several factors, including normal hormone fluctuation, pregnancy, and vaginal hygiene (2). Lactobacillus species reinforce the defense against pathogens and prevent colonization by opportunistic microbes. Higher levels of estrogen promote the maturation, proliferation and accumulation of glycogen in the vaginal epithelial cells (VEC). The acidic environment created by glycogen metabolism is vital for Lactobacilli to thrive (1).
Vaginitis is often caused by vulvovaginal candidiasis (VVC), in fact, VVC is considered the second most common cause of vaginitis. Although the rate of VVC is common, the causes of occurrence and recurrence are often unclear. At least 75% of all women suffer from Candida infection once in their lives. Over 85% of VVC cases are principally caused by C. albicans, with the remaining cases attributed to C. parapsilosis, C. tropicalis, and C. glabrata (3). Two different kinds of candidiasis infection occur in the vulvovaginal area: a more severe “classic” infection with occasional recurrences, and a “cyclical” type (4). The most common signs of VVC are pruritus, burning, dyspareunia, vaginal and vulvar erythema (4).
Recurrent vulvovaginal candidiasis (RVVC) has been defined as at least 3 symptomatic episodes in the previous 12 months (5). RVVC can be caused by a vaginal reservoir of Candida (5). Excessive vulvovaginal hygiene care with products including soaps, douches and creams may predispose to fungal infections by contributing to vaginal milieu disruption. Similarly, several risks factors, including immunosuppression, vaginal immune defense deficiencies, antibiotic therapy, and fluctuations in estrogen levels, have been detailed to be implicated in RVVC (6).
The objective of this study was to correlate clinical finding and lab culture method used in identification of fungal communities, and to examine the relationship of Lactobacillus and Candida species in patients with recurrent vulvovaginal candidiasis.
Materials and methods
Vaginal samples were collected as part of a prospective data bank creation to study vaginal conditions (ClinicalTrials.gov). The study was approved by the Institutional Review Board (protocol# L13-054) at Texas Tech University Health Sciences Center, TX, USA. The samples were obtained from the middle of the vagina using standardized cotton swabs (7, 8). The vaginal specimens were placed into 1 ml of physiological solution (phosphate-buffered saline) and stored at -80ºC.
Fungal cultures
Sabouraud’s Heart Infusion Agar (SABHI) and Mycobiotic Agar (MA) were used for fungal cultures. The specimens were submitted to the laboratory immediately upon retrieval, and the samples were protected from temperature fluctuations. The SABHI was stored in a refrigerator and brought to room temperature prior to inoculation. The entire surface of the agar slant was swabbed and the specimen was pushed lightly into the agar. The plate was incubated at 25-30°C for 30 days.
The MA powder was suspended in distilled water and then autoclaved at 118 °C for 15 minutes. The medium was dispensed into the plates. The specimen was inserted into the agar and incubated at 22-25 °C. The growth was observed to determine typical colonial morphology of the fungi for four to six weeks.
To perform the wet mount potassium hydroxide (KOH) preparation, the specimen was placed on a glass slide and 1 drop of 20% KOH was added on the top. Then, a glass cover slip was placed on the top of the specimen. The resultant fungal structure was examined with a microscope.
Lactobacilli identification
The relative concentration of the vaginal flora was determined via a real-time polymerase chain reaction (qPCR), as described previously (2, 9, 10). A qPCR assay was performed to identify the presence of Lactobacillus species including L. crispatus, L. gasseri, L. iners, and L. jensenii. qPCR analysis, RNA preparation and comparative ∆∆Ct method were described in previous study (2, 9).
Statistical Analysis
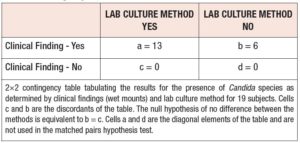
The sample data allow for comparing the sensitivities of the lab culture method and the clinical finding method. Since only patients with candida infections were considered, specificity and other test measures could not be compared. As the clinical finding and lab culture methods were used on the same subjects, the data represent dependent or matched-pairs samples as shown in Table 1.
The comparison of proportions for matched-pairs data can be accomplished with the exact McNemar’s test. The exact McNemar test is a binomial test with two-sided p-value computed as
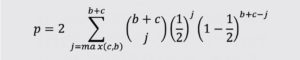
Results and discussion
Vaginal samples obtained from patients with confirmed Candida infections showed the presence of C. albicans, C. dubliniensis, C. glabrata, and C. parapsilosis. Clinical findings suspected all the patients colonized with Candida, while the lab culture method detected Candida species in approximately 68.5% of patients. C. albicans and C. dubliniensis were the most abundant species in nearly 75% of patients with Candida infection. The observed 22% differences between two methods may results from some patients applying creams or using douches for their genital area prior to visiting our clinic. Other possibilities are incomplete sampling or problems related to transportation or storage.
VEC provide a microenvironment that conserves vaginal health by nurturing Lactobacilli through the maintenance of innate and attained immunity mediators (11). Moreover, VEC are the foremost barrier against C. albicans. These cells express a diverse pattern recognition receptors by way of dectin-1, lectin like-receptor families and several of the Toll-like receptors (11). Amongst others, dectin-1 is responsible for the activation of two pathways (Src-Syk-CARD9 (caspase recruitment domain-containing protein 9) and NF-kβ), which transcribe powerful antifungal cytokines including IL-1β, IL-6 and IL-23 β-glucans (11). In addition, CARD9 contributes to the signaling of dectin-2 and macrophage-inducible C-type lectin, which identify C. mannans and explain fungal infections in women (12). Identification of 1-linked and 3-linked β-glucans by dectin-1 has been reported to be one of the crucial pathways for fungal recognition. It has been shown that dectin-1 deficient mice have a higher predisposition to C. albicans infections (12).
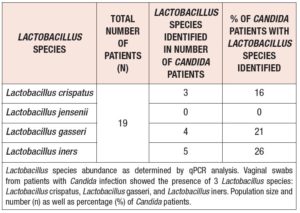
Vaginal swabs from patients with Candida infection showed the presence of L. crispatus, L. gasseri, and L. iners, with L. iners being identified as the most dominant species (Table 2).
The difference among the Lactobacillus species in patients with Candida infections may be the result of a varying level of ability to confer protection against various Candida species. Additionally, women that are deficient in mannose-binding lectin are more susceptible to recurrent C. albicans infections (13).
Women diagnosed with bacterial vaginitis are frequency treated with antimicrobial therapy which contributes to fungal vulvovaginitis due to alterations in the commensal microorganisms of the vagina. Lactobacillus species utilization in prevention and/or treatment of vaginal candidiasis is a promising technique. Some studies have reported efficacy in using L. rhamnosus and L. fermentum to colonize and restore urogenital flora to women with a history of yeast vaginitis. Studies have shown the ability of Lactobacilli probiotics mixed with lactoferrin to reduce vaginal candidiasis recurrence. Lactobacilli could also be utilized in the fight against pathogenic bacteria. Recently, Lenzmeier et al. showed that L. gasseri prevented sepsis caused by opportunistic pathogen Pseudomonas aeruginosa in patients that suffered severe burns, and other immunocompromised individuals (14).
Numerous women with the symptoms described above, use over-the-counter antibiotics to empirically treat their vaginal candidiasis. It is well established that Candida in the vagina is only discovered in roughly half of the patients presenting with classic symptoms (15). The presence of vaginal symptoms related to fungal infection with a positive microscope suspicious at the wet mount examination are a stimulating finding in our study. These findings in our population of patients with RVVC, will make it possible, with a 68.5% degree of confidence, for the clinician and the patient to be treating the correct condition with respect to RVVC. The importance of microbiological culture in the identification of specific fungi should be emphasized.
In patients with VVC, the ability to clinically diagnose and administer an early treatment protocol is important in preserving the patient’s quality of life. It is reassuring that fungal colonization did not interfere in our population with the Lactobacillus spp., allowing them to continue providing usual protection against other associated pathogens.
Conclusion
Lactobacillus species were found in presence of fungi colonies within patients with recurrent vulvovaginal candidiasis. While all patients exhibited signs and symptoms of recurrent vulvovaginal candidiasis, we found that only 68.5% of the cultures were positive. The importance of microbiological culture in the identification of the specific fungi should be emphasized.
ABBREVIATIONS
CARD9 Caspase Recruitment Domain-Containing Protein 9
KOH Potassium Hydroxide
MA Mycobiotic Agar
qPCR Real-time Polymerase Chain Reaction
RVVC Recurrent Vulvovaginal Candidiasis
SABHI Sabouraud’s Heart Infusion Agar
VEC Vaginal Epithelial Cells
VVC Vulvovaginal Candidiasis
ACKNOWLEDGEMENTS
We are thankful to Ailena Mulkey, Evangelina Santiago, Jammie Holland and Erik Wilkinson for their support. We are grateful to Dr. Scott Gygax their assistance in the quantification of vaginal flora by qPCR analysis.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
PATIENT CONSENT
The consent forms were obtained from all the patients.
FINANCIAL DISCLOSURE
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONS
Dr. Gary Ventolini consulted and obtained the consent. GV, KG, PG and JG designed the study. GV, KG, JG, RA, PG and JNG contributed in writing and editing of this short communication. All authors have approved the submitted version.