Introduction
Abnormal uterine bleeding (AUB) occurs in approximately 5% of postmenopausal women. Since 7 to 9% of AUB cases are due to endometrial cancer, the primary aim of the evaluation in all post-menopausal women with AUB is to exclude malignancy. (1,2)
Ovarian carcinoma is the third most common gynecologic cancer. Epithelial tumors account for 95% of ovarian tumors; the other 5% are germ cell, sex cord and stromal tumors. (3)
In 2014, the WHO reclassified epithelial ovarian carcinomas, indicating five histologic types: (4)
- high-grade serous tumors (70%)
- low-grade serous tumors (5%)
- mucinous tumors (20%)
- endometrioid tumors (10%)
- clear cell tumors (4 to 10%)
Case report
We report the case of a 75-year-old female patient who was referred to the Gynecologic Endocrinology Section at Hospital de Clínicas José de San Martín with a four-month history of episodes of abnormal uterine bleeding. The patient was referred with a previous transvaginal ultrasound showing endometrial thickening (an endometrial thickness of 7.4 mm); she had also had a previous office hysteroscopy with removal of 2 polyps, reported as benign. Other tests provided by the patient included trophic annual pap smears from the previous five years plus one recently performed one, and hormonal measurements showing elevated (for her age) serum levels of estradiol and suppressed gonadotropins.
As regards to the patient's history, she became menopausal at the age of 45 and did not receive hormone replacement therapy. She had three pregnancies and three normal deliveries. She currently has controlled hypertension (blood pressure 130/80 mmHg) and obesity (a body mass index of 32).
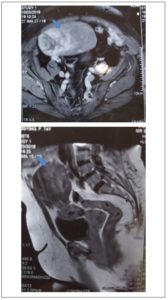
The gynecologic examination performed at the patient's visit to the Gynecologic Endocrinology Section revealed trophic genitalia and leukorrhea, with normal cervix. The breast examination was normal. On abdominal palpation, a 10-cm solid, mobile mass was detected in the right quadrant. Therefore, an abdominal ultrasound was ordered, which showed a 10-cm highly vascularized mass in the right iliac fossa. This finding was confirmed by a magnetic resonance imaging (MRI) scan of the abdomen and pelvis, which showed a solid, heterogeneous 92 x 134 x 81 mm right adnexal mass, extending to the abdominal cavity. (Figure 1).
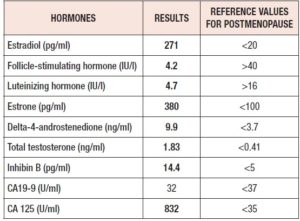
Hormonal blood tests were repeated, confirming hyperestrogenism and also revealing hyperandrogenism. Tumor marker measurements showed elevated CA-125 levels. (Table 1).
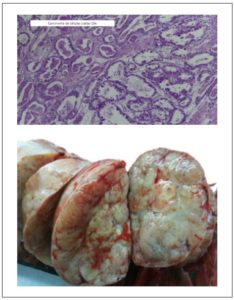
After reviewing the results of the tests performed, an exploratory laparotomy was indicated for suspected hormone-producing malignant adnexal tumor. The patient was referred to the Gynecologic Oncology Unit, and was submitted to an exploratory laparotomy. She underwent an Total Abdominal Hysterectomy with Bilateral Salpingo-oophorectomy (TAH/BSO), pelvic and para-aortic lymphadenectomy, omentectomy and peritoneal biopsies. The pathology report revealed on gross examination: right ovary replaced by a 15 x 9 x 8 cm solid mass; on microscopic examination: clear cell carcinoma, with a tubulocystic architectural pattern and focal solid and papillary areas; areas of adenofibroma; negative nodes and peritoneal biopsies; negative peritoneal washings; endometrial polyp. (Figure 2).
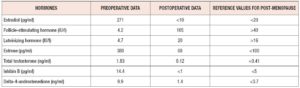
The patient subsequently completed six courses of adjuvant chemotherapy (paclitaxel and carboplatin). She returned to the Gynecologic Endocrinology Unit nine months after surgery and had follow-up hormone measurements performed, which showed results consistent with her age. (Table 2).
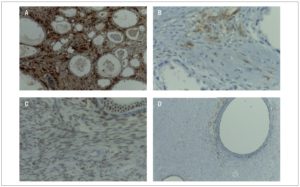
Since the patient had presented with AUB and abnormal hormonal blood tests, and the pathology report revealed clear cell carcinoma, immunohistochemistry (IHC) of the surgical specimen was ordered. The IHC technique was used for analyzing the expression of calretinin, inhibin, estrogen, progesterone and androgen receptors. Focal positivity for calretinin and inhibin was seen in luteinized cells and in theca cells of the adenofibromatous areas, as well as in small areas of the clear cell carcinoma.
Positivity was found for estrogen and progesterone (estrogen: moderate 40% intensity; progesterone: strong 90% intensity) in the adenofibroma areas. IHC was negative for androgen receptor. (Figure 3).
The histopathological findings are consistent with a clear-cell carcinoma with areas of fibroadenoma and functioning stroma.
Discussion
Hormone production by ovarian tumors is mainly related to sex cord-gonadal stromal tumors, and less frequently to other tumors such as gynandroblastoma, gonadoblastoma, carcinoid tumor, epithelial tumor with functioning stroma and metastatic tumors. (5)
Among epithelial tumors, functioning stroma is usually seen in mucinous and endometrioid types, although some cases of clear cell carcinomas, similar to our case, have been reported. These epithelial tumors contain hormone-producing lutein cells, theca cells or both. (6-8)
This non-steroid-producing ovarian tumor is detected by clinical manifestations of endocrine hyperactivity and confirmed by the identification of luteinized and theca-like cells in the stroma. Demonstration of the presence of hormone receptors confirms that tumor cells can activate the surrounding stroma to produce steroid hormones and that these hormones, in turn, can stimulate the growth of the tumor. These findings suggest an intricate interplay between the tumor and the adjacent ovarian stroma. (9)
In addition, even though we were unable to perform immunohistochemistry of steroid enzymes, it has been previously reported that lutein cells of ovarian tumors with functioning stroma have low cytochrome P450 enzyme activity. (10) For this reason, we think that the high estrogen levels in this patient were not due to production by the tumor itself, but rather the result of intraovarian and peripheral aromatization of androgens. This peripheral conversion in our patient might have been enhanced by the presence of obesity. (11)
A potential interaction of the stroma with ovarian carcinoma is most likely and opens up possibilities for a therapeutic strategy associated with the use of antisteroidogenic drugs (aminoglutethimide, azoles,etc.).
Conclusions
Postmenopausal patients with AUB should be evaluated for the presence of steroid-producing ovarian tumors once endometrial cancer has been ruled out, even if uterine bleeding is the only clinical expression of the tumor, with no associated signs of hyperandrogenism.
Conflict of interest statement
The authors state they have no conflict of interest.