Background and Purpose
In in vitro fertilization (IVF) cycles, various ovarian stimulation protocols are used with the aim of obtaining a sufficient number of oocytes. However, these protocols often do not achieve the desired result. Patients with low ovarian reserve and insufficient response to ovarian stimulation, unfortunately, constitute the most numerous cohorts in IVF clinics. Current practice clearly demonstrates that in more than one-third of stimulated cycles there is a “poor” response (1). Depending on the evaluation criteria used, the observed frequency of reduced ovarian response or “poor ovarian response”, as it is commonly referred to in the literature, ranges from 5.6% to 35.1% of cycles (2-8). However, in IVF, the number of oocytes and embryos produced during ovarian stimulation is a key factor contributing to the success of the treatment, as it determines the possibility of subsequently selecting optimal morphology embryos for transfer.
Poor ovarian response to gonadotropin stimulation leads to frequent cancellation of cycles (up to 75%) and low rates of pregnancy (5-15%) (9-15). This problem is primarily due to increasing rates, in treated populations, of women of older reproductive age (after 35-38 years), who show a physiological decrease in ovarian reserve and a high frequency of embryo aneuploidy (16). Other factors that cause poor ovarian response are: genetically determined causes, previous surgery on the ovaries, and the presence of gynecological diseases (genital endometriosis, pelvic inflammatory disease, premature ovarian depletion) (9).
It has been found that in some patients with poor IVF cycle outcomes, the administration of adequate doses of gonadotropins does not lead to maturation of more than 3 follicles, or even fails to stimulate their growth. In recent years, numerous attempts have been made to modify IVF protocols. However, the problem of overcoming infertility in the presence of a “poor ovarian response” to stimulation is still far from finding a scientific and practical solution. For example, there is no “ideal protocol” of stimulation, providing a noticeable improvement in ovarian response, any approaches to the preparation of such patients are not clearly justified (3, 17, 18).
Currently, in the literature there are many scientific papers focusing on methods to improve the efficacy of IVF procedures in patients with “poor” response. Strategies in these cases include both approaches focusing on adjuvant therapy before controlled ovarian stimulation (COS), and approaches focusing directly on the choice of stimulation protocols, and the type, doses and modes of use of gonadotropins. However, there is currently no definitive position regarding patients with a perceived “poor ovarian response”. Clinicians, in numerous attempts to help patients, may offer various options striving to obtain preliminary “effects on the ovaries” through the use of bioidentical estrogens and progesterone in the mode of cyclic hormone therapy (19), growth hormone drugs (20), and androgens or androgen-like supplements (21,22).
To realize the reproductive function of patients with a “poor” response, it is necessary to understand the cause of reduced ovarian reserve, particularly in patients of young reproductive age. It is suggested that “poor” response may be partly due to a shortened follicular phase and the limited ability to be selected in the growth of a significant cohort of follicles during folliculogenesis. It is believed that in the ovarian stimulation cycle there are noticeable differences in the size of follicles in the early follicular phase, which leads to a low number of mature oocytes and a low potential for their fertilization, which in turn negatively affects the number of transferred embryos and the probability of conception (23, 24). Attention is drawn to studies of the use of estradiol (E2) drugs in the cycle preceding the one in which patients receive superovulation stimulation with GnRH antagonists (25, 26). The main idea behind this approach is that endogenous estradiol is involved in the negative feedback mechanism in the reproductive axis, which is realized through inhibition of GnRH secretion and, accordingly, gonadotropin response. This relationship is thought to be maintained even at low physiological ranges of blood E2 levels (26).
Studies have shown that use of natural negative feedback of the hypothalamus-pituitary-ovary axis, caused by E2 pre-priming, can effectively prevent intercyclic increases in the concentration of follicle-stimulating hormone (FSH), lead to more coordinated follicular growth and therefore make it possible to obtain more mature oocytes (25, 27, 28).
In addition, some authors in recent years have suggested that using a GnRH antagonist in the follicular phase before the introduction of gonadotropins can lengthen the follicle recruitment phase, thereby allowing more follicles to grow by suppressing endogenous FSH and preventing the formation of the corpus luteum, without compromising ovarian stimulation in the event of poor response (29, 30).
Cakmak et al. (2) and later Lee et al. (8) proposed synchronizing follicle pool growth by applying estrogen priming during the late luteal phase and then injecting a GnRH antagonist for 7 days into the early follicular phase prior to gonadotropin stimulation, focusing on the heterogeneity of follicle sensitivity to gonadotropins during these days of the cycle. These authors reported improved ovarian response.
The objective of the present study was to evaluate the outcomes of IVF cycles in relatively young patients with poor response to COS (according to the Bologna criteria) (31), who underwent a standard protocol supplemented with pre-priming with transdermal gel having a 17-β estradiol concentration of 0.06% (Estrogel®, Besins Healthcare SA, Belgium) in a real-world setting.
Methods
An open, comparative, prospective, non-interventional study was conducted involving 8 clinics of the Mother and Child Group (Moscow, Ufa, Samara, St. Petersburg) in the period of 2018-2019, after appropriate examination and approval of the protocol by an independent interdisciplinary committee for ethical examination of clinical trials.
During the study, the following criteria for inclusion/non-inclusion/exclusion of patients were observed:
Inclusion criteria: age 25-35 years; negative pregnancy test performed at inclusion in the ECO program; a body mass index of 19-29 kg/m2; an anti-Müllerian hormone (AMH) level of 0.5-1.5 ng/ml, an E2 level of at least 500 pg/ml on the day of trigger prescription in previous protocols; number of antral follicles: less than 5 on days 2-3 of the cycle or more than 1 poor response when superovulation was performed in previous cycles, patient’s informed consent to participate in the study; the decision on prescription of application of priming with estradiol hemihydrate until inclusion in the study.
Non-inclusion criteria: any contraindications to the use of estradiol hemihydrate; male factor infertility.
Exclusion criteria: progesterone level of more than 1.5 ng/ml on the day of trigger administration; endometrial thickness less than 7 mm on the day of trigger administration; embryos with morphology graded less than 3BB according to the Gardner classification on the day of transfer.
After assessment of the inclusion/exclusion criteria, the study included 258 patients with previous poor ovarian response who underwent COS with pre-priming with estradiol hemihydrate (study group) and 126 patients with poor response who underwent COS without pre-priming (control group).
All therapy procedures were performed with the patients' consent to the treatment protocol. In all the study group patients, priming with estradiol hemihydrate transdermal gel (Estrogel®, Besins Healthcare SA, Belgium), at a dose of 3 mg, began 12-14 days before the expected start of the cycle during which patients were due to undergo stimulation, and continued until day 3 of the subsequent cycle. Recombinant FSH follitropin beta (Puregon®, Organon, Netherlands) or menotropin (Menopur®, Ferring GmbH, Germany) at a dose of 225-250 IU was prescribed from days 2-3 of the cycle after determining the level of FSH, E2, during stimulation the dose did not change. The GnRH antagonist ganirelix (Orgalutran®, NV, Organon, Netherlands) 0.25 mg was prescribed from day 5 of superovulation stimulation in a fixed mode until the day of trigger administration inclusive. Recombinant or urinary chorionic gonadotropin (Pregnil® 10,000 IU, NV Organon, Netherlands; or Ovitrel® 250 mg, Merck Serono S. p. A., Italy) was administered when 1 or more follicles with a diameter of 17-18 mm were reached.
All patients underwent dynamic transvaginal ultrasound (on average once) on days 2-3. Against the background of superovulation, on the day of the appointment, a trigger was used to determine the serum level of progesterone. At levels higher than 1.5 ng/ml patients were excluded from the study. Ovarian puncture was carried out 35-36 hours after the injection of the trigger; in all cases, two-lumen systems for oocyte sampling were used. To support the luteal phase, micronized progesterone (Utrogestan®, Besins Healthcare SA, Belgium) was administered at a dose of 400 mg intravaginally from the day after the puncture. Transfer of 1 or 2 embryos was performed on day 5.
The main criterion for evaluating efficacy was frequency of implantation (frequency of biochemical pregnancy). Biochemical pregnancy was diagnosed with a serum β-HG test 12 days after embryo transfer.
The duration of COS before the injection of the trigger, the total dose of gonadotropins used, the number of follicles in the course of COS, the levels of E2 and progesterone on the day of the trigger injection, the number of retrieved oocytes and embryos, the number of embryos with good morphology on day 5 according to the Gardner classification, the incidence of clinical and ongoing pregnancy, and endometrial thickness on the day of embryo transfer were all evaluated as well. Clinical pregnancy was determined by visualization of the fetal egg using transvaginal ultrasound at 3 weeks after embryo transfer. Progressive pregnancy was determined at 7-8 weeks of pregnancy by the presence of an embryo in the fetal egg with a regular heart rate.
The main software used for statistical analysis was the IBM SPSS 22 statistical package. Statistical analysis consisted of research and descriptive methods, with the required 80% power of the study (beta error of 20%) and allowable alpha error of 5%. Demographic data of patients and baseline data were presented as rates or percentages, or using mean (standard deviation), median (interquartile range), minimum and maximum, depending on the type of variable. To test the homogeneity of the study groups at baseline, as well as after treatment, null hypotheses (absence of differences between the groups) were tested using Student’s t-test (for interval indicators with normal distribution in the study population), the Mann-Whitney test (for ordinal indicators or for interval indicators with a distribution other than normal) or the c2 test (for qualitative characteristics). In case of statistically significant differences between the groups, the magnitude of the differences between them was estimated.
Results
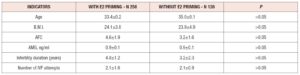
At the time of inclusion in the study, the groups of patients did not differ in age, body mass index, duration of infertility, number of antral follicles, AMH level, or number of previous IVF attempts. The main characteristics of the patients included in the study are presented in table 1. In patients with estradiol hemihydrate treatment in the luteal phase, the level of FSH on days 2-3 of the cycle was significantly lower compared with the mean basal level of FSH in patients without priming (6.8±3.2 vs. 9.0±2.8 mIU/mL, p<0.05), and the level of E2 was significantly higher (154±62.2 ng/ml vs. 46±18.9 ng/ml, p<0.05). These results were not surprising since the mechanism of action of estrogen priming is suppression of gonadotropin production by the feedback mechanism.
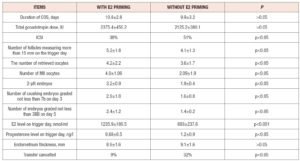
In the cycles of the standard protocol with GnRH antagonists and no E2 priming (control group), cycles were cancelled in 32% due to inadequate folliculogenesis or poor embryo morphology, whereas in the study group, the cycle cancellation rate was only 9% (p<0.05), table 2. The duration of COS did not differ between the groups: 10.8±2.8 days in the study group and 9.9±3.2 in the controls (p>0.05). Intracytoplasmic sperm injection (ICSI) was performed in 38% of fertilization cases in the E2 priming group compared with 51% in the control group (p<0.05). The total dose of gonadotropins did not differ between the compared groups (p>0.05).
Significantly higher peak E2 levels were achieved in the E2 priming group compared with the control group (1,235.9+189.5 vs. 693+237.6 nmol/ml, p<0.001). Moreover, in the study group, a greater number of oocytes was obtained by ovarian puncture, and there was also a tendency to obtain more and better-quality embryos in the group with E2 priming. Table 2 compares stimulated cycle parameters between the groups of patients. None of the patients reported adverse events associated with the prescription of estradiol hemihydrate.
Transfer of obtained embryos was performed in 91.1% of cases (235 patients) in the study group, and in 68% of cases (85 patients) in the control group, p<0.05. The clinical results of the study show a higher implantation capacity (biochemical pregnancy), and higher rates of clinical and progressive pregnancy in the group of patients with estradiol hemihydrate priming compared with the control group (table 3). However, due to the small number of patients studied, the statistical significance of these indicators requires further clarification.
Discussion
The results of this observational study largely correspond to those of other studies reported in the literature (8, 25, 32). The accumulated experience of IVF cycles clearly demonstrates the need to choose effective strategies in patients with a “poor” response to COS. When using adjuvant therapy, namely treatment with E2 drugs in the luteal phase before COS in the protocol with GnRH antagonists, we obtained a significant decrease in the number of cancelled cycles, while obtaining significantly more oocytes suitable for fertilization. Similar data were demonstrated in the meta-analysis of 8 studies (468 respondents) by Reynolds (32), and in 7 studies (450 respondents) reported in the systematic review by Chang and Wu (25). However, they noted that with an overall increase in the number of suitable oocytes, the duration of stimulation increases, albeit not significantly, which was not found in our observation. It is noteworthy that at the time of starting superovulation stimulation, the size of the antral follicles after estrogen priming was significantly larger than in the control group (5-7 mm vs 3-4 mm, p < 0.05), which allowed us to complete the superovulation stimulation within 10-13 days in all patients. Notably, some of them had a history of previous IVF programs with prolonged stimulation for 14-17 days with slow follicle growth. In addition, the analyzed data indicate a clear tendency to increases in the frequency of implantation and of pregnancy in cycles with E2 pre-priming compared with the standard COS protocol. In the study group, the implantation rate was 28% compared with 17% in the control group, while the frequency of clinical pregnancy in the study group and the control group was 32% and 21%, respectively, thus a significantly higher rate in patients with an initially high probability of cycle cancellation and lack of embryo selection and transfer, on the principle of “as is”.
The concept of estrogen priming was first proposed by Fanchin et al. and it was based on the assumption that synchronizing the growth of early antral follicles would optimize COS and improve cycle outcomes (27, 28). It has been shown that asynchronous follicle growth, arising from differences in the sensitivity of each follicle to FSH and the gradual increase in FSH from the middle of the luteal phase, dramatically undermines efforts to obtain good oocytes, and that GnRH agonists and oral contraceptives, usually used for uniform selection of the follicle cohort (9), quite often cause mediated hyperthermia of hormonal secretion by the ovaries (33-35). In contrast, an advantage for patients with a “poor” response is the use of E2 drugs in the luteal phase of the preceding stimulation cycle (36-38). In this study, we compared the protocol with priming with estradiol hemihydrate prior to COS with the standard protocol using GnRH antagonists, analyzing important clinical and embryological parameters of the IVF cycle. The objective of this priming was to synchronize the growth of follicles and obtain more oocytes suitable for fertilization, and thus to increase the chances of pregnancy in patients with a “poor” response. The benefit of continued priming during stimulation, to prevent the surge of endogenous gonadotropins and subsequent suppression of FSH receptors in granulosa cells after E2 reduction, is also discussed in literature (25, 39, 40). At the same time, prolongation of E2 use against the background of gonadotropin use, as suggested and analyzed by some authors, increases the impact of FSH on follicle granulosa receptors and indirectly improves the quality and potential of oocytes. This assumption was based on the well-known fact that follicle growth and granular cell proliferation are enhanced by estrogens and FSH (41). Researchers indicate that only estradiol induces proliferation of FSH receptors in granulosa cells, and that continued use of E2 drugs with the initiation of gonadotropins maximizes expression of FSH receptors in granulosa cells and LH / HG receptors in granulosa and theca cells (42).
Conclusion
The study suggests that estrogen priming may boost the chances of increasing the number of oocytes obtained through subsequent stimulation with gonadotropins. The ability to regulate the uniform growth of follicles and obtain more mature oocytes, even in the context of a small total number, is an advantage in patients with initially low ovarian potential. In this complex, quantitatively dependent process, there is a “competition” for each oocyte that can be realized in pregnancy. In addition, in 24% of patients with estrogen priming, according to the data presented in the study, which do not contradict similar studies performed earlier, it was possible, after transfer, to vitrify remaining embryos suitable for further use. For comparison, in the control group of such patients the rate was only 11%.
The observed IVF result may be a consequence of both follicle growth synchronization and stimulation of E2 FSH receptors in granulosa cells. On the whole, priming does not contribute to a reduction of hormonal load, as the total dose of gonadotropins in the two groups did not differ. According to some authors, a slower and more coordinated process of follicle growth may require an even higher dose of gonadotropins.
Beyond the scope of this study, with regard to compliance with the treatment and tolerability of estradiol hemihydrate, no cases of adverse events or refusal of treatment were observed. The main postulate underlying the choice of this drug was the known relative safety of the transdermal route of estrogen administration; indeed, the fact that there is no effect of “primary passage through the liver” helps to reduce the systemic risk of this hormone therapy. In addition, the dosage form of the transdermal gel with a 17-β estradiol concentration of 0.06% — a bottle with a dispensing pump — ensures accuracy and convenience of estradiol dosing, which is an important aspect when performing estrogen priming.
Acknowledgments. No financial support was received for the work being published.
Conflict of Interest Statement. The authors declare that there is no conflict of interest.