Introduction
The use of oocyte donation in the treatment of infertility has increased constantly in recent years (1). The main indications for the use of egg donation, proposed by the American Society for Reproductive Medicine, include primary ovarian failure, prevention of genetic diseases in offspring, decreased ovarian function, persistence of poor oocyte/embryo quality, and advanced reproductive age (2).
Oocyte donation is generally accomplished by synchronizing the infertile woman's menstrual cycle with a donor ovarian stimulation cycle (3, 4). Oocyte donation IVF generally has the highest live birth rates (5) of any IVF treatment in the United States (6).
The most common limitations with fresh cycles are related to donor availability, cost, and the need to synchronize the donor’s schedules and to achieve transfer within a certain period of time. Vitrification offers a chance to overcome the need for recipient-donor synchronization and allows a case to be assigned a specific number of oocytes, simplifying the process and also lowering the cost and efficiency of oocyte donor programs (7). A mild IVF cycle refers to the method wherein Follicle Stimulating Hormone (FSH) or Human Menopausal Gonadotropin (hMG) is administered at lower doses (up to 150 IU/day) and/or for a shorter duration in a GnRH antagonist co-treated cycle; the goal is to collect between 2 and 7 oocytes per cycle [(8). The aim of this study was to compare the laboratory and clinical outcomes of the use of equal numbers of oocytes from fresh and vitrified donor cycles.
Methods
We performed a retrospective, comparative and cross-sectional study of treatments administered to patients participating in an egg donation program at the IECH Fertility Center (January 2014 to December 2018) in Monterrey, México. From our oocyte donation program, we selected a specific group of donors to undergo mild ovarian stimulation in order to obtain a specific number of oocytes during the fresh cycle. A total of 40 patients met the inclusion criteria and underwent embryo transfer. Group 1 (n=27) received vitrified eggs obtained exclusively from ovarian stimulation cycles within our oocyte donation program and group 2 (n=13) received fresh eggs from synchronized recipient-donor cycles. Cycles with severe male factor were excluded from the analysis.
Egg donors
The egg donation program includes patients aged between 18 and 25 years, with a body mass index of between 18.5 and 24.9 kg/m2. In accordance with American Society for Reproductive Medicine (ASRM) gamete and embryo donation recommendations (9), in addition to undergoing an assessment protocol that includes a clinical evaluation and complete physical examination, donors undergo serum tests to exclude infectious diseases (HIV, hepatitis B and C, VDRL, STORCH), substance abuse, and other diseases (TSH, PRL and HAM).
Mild IVF stimulation
Long-agonist protocols were used for all donors and 112.5 UI of recombinant FSH for 8 days of stimulation according to the ISMAAR criteria (8).
Vitrification and warming
All oocytes classified as metaphase II were vitrified using the Cryotech® vitrification and warming kit (10).
Laboratory parameters and reproductive outcomes
In the group of fresh oocytes, all the oocytes were inseminated or injected 5 hours after retrieval, depending on the seminal characteristics of the couple. In the vitrified group, the survival rate was assessed 2 hours after thawing to perform ICSI.
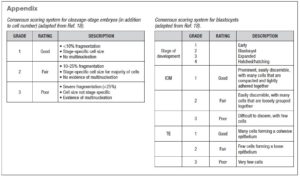
Fertilization, cleavage rate and blastocyst yield were assessed. Single-embryo or double-embryo transfer was performed according to clinician and patient preference. The quality of the blastocysts was classified according to the Consensus of the ALPHA company (11) (see supplementary material). Primary laboratory outcomes (fertilization, cleavage, blastocyst development and implantation rates) and secondary clinical outcomes (clinical pregnancy per embryo transfer, miscarriage rate, live-birth rate and multiple pregnancy rate) were assessed.
Statistical analysis
The database was collected for statistical analysis, evaluating central tendency with standard deviation (SD) for further analysis with the Student T and Kruskal-Wallis tests, depending on the data normality, and Fisher’s exact test to show differences between the two groups and their frequencies. The GraphPad Prism Version 8 program was used, and p values <0.05 were considered significant.
Results
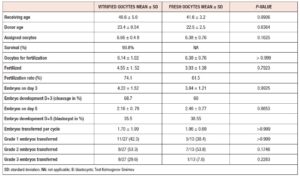
As shown in table 1, demographic characteristics did not show statistical differences. Patients in both groups were assigned a median of 7 eggs from the donation program. In group 1, the survival rate was 93.8%. In group 2, 23% were treated with ICSI, and the rest with IVF due to good sperm parameters.
The fertilization rate was 74% versus 61% in groups 1 and 2, respectively. The cleavage rate was 68% for group 1 vs 60% for group 2 (p=NS). Blastocyst yield was 2.18±0.78 (35%) versus 2.46±0.77 (38%), with no significant difference.
In group 1, 43% of the embryos transferred were top quality, versus 38% in group 2 (see table 1). Poor embryos were transferred when they were the only ones available (30% in group 1 and 8% in group 2).
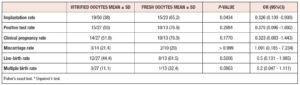
The pregnancy rate was 55% in group 1 and 77% in group 2 (p=NS) (Table 2). The miscarriage rate was similar between the groups: 21% vs 20%, respectively. The live birth rate was 44% in group 1 versus 62% in group 2. The live birth weights were similar: 2,545 ± 713 gr vs. 2,512 ± 502 gr. Twin pregnancies (32% vs.10%) were more frequent in group 2 than in group 1. No triplets were conceived.
Discussion
Indications to cryopreserve everything have substantially expanded in recent years; studies have shown vitrified frozen embryo transfer to be associated with equal or higher implantation and pregnancy rates compared with fresh embryo transfer (5, 12, 13). Vitrification/warming has been resulting in improved oocyte, cleavage-stage embryo and blastocyst survival rates, and also improved clinical outcomes; however, the evidence is not completely in favor due to a serious risk of bias in the studies (14).
In previous comparisons of vitrified versus fresh cycles, the number of oocytes yielded and compared was the main obstacle to analysis, even for prospective studies and/or randomized clinical trials. Therefore, to optimize the statistical analysis, we performed mild stimulation cycles retrieving a specific number of oocytes.
We found no differences in laboratory outcomes, however, blastocyst yield was lower than the average reported in the world literature (10), probably due to the small number of oocytes utilized (15). A statistically significant difference was observed in implantation rate between the groups, with an IR of 65.2% for the fresh vs 38% for the vitrified group (p= 0.0434, OR= 0.326 [95%CI=0.109-0.930]).
Clinical pregnancy rate, implantation rate, live-birth rate and multiple pregnancies did not show statistical differences, in line with evidence previously reported by Trokoudes et al. (13), Domingues et al. (16), and García et al. (17). Differences in frequencies between groups were noticeable, but did not reach statistical significance, possibly due to the small sample size. (16), and García et al. (17). Differences in frequencies between groups were noticeable, but did not reach statistical significance, possibly due to the small sample size.
Conclusions
This study is a small trial for evaluating whether vitrification can be considered a next step in donor egg cycles, in order to avoid the difficulties currently associated with fresh cycles. Nevertheless, the reproductive outcomes of the two methods should be similar in order to offer candidates for donor eggs programs the best possibility of achieving a pregnancy as soon as possible. Although size of the present sample is a limitation of this study, a small number of vitrified eggs may not deliver the same probability than the same number of fresh oocytes.
DECLARATION OF INTERESTS
The authors do not report any conflict of interest.
FINANCING
The authors do not report any source of financing to carry out this article.