Introduction
Vitamin D is a fat-soluble vitamin and its main natural source is cutaneous synthesis during exposure to solar ultraviolet (UV) rays. Dietary sources of vitamin D include cod liver oils and fatty fish, as well as fortified foods. 1 There are two forms of vitamin D: vitamin D3 (cholecalciferol), derived from exposure to sunlight, and vitamin D2 (ergocalciferol) which can be produced in the skin or ingested in the diet. The two forms have minor differences in bioactivity. 2 In order to become bioactive, vitamin D undergoes a first hydroxylation in the liver, into 25-hydroxyvitamin D [25(OH)D] or calcidiol, and a second one in the kidneys and other tissues, into 1,25-dihydroxyvitamin D or calcitriol.
The main circulating form of vitamin D is 25(OH)D and its levels are the best laboratory indicator for vitamin D sufficiency. The optimal serum level of 25(OH)D is still debated among experts. The Institute of Medicine suggests that serum 25(OH)D levels of 20 ng/mL (50 nmol/L) are sufficient for most individuals,3 whereas the European Menopause and Andropause Society and the Endocrine Society define vitamin D insufficiency as 25(OH)D concentrations between 20 and 29.99 ng/mL (50 and 74.99 nmol/L), and vitamin D deficiency as concentrations below 20 ng/mL. 4,5
Despite the debate, vitamin D deficiency is a global problem, affecting countries with different latitudes and different availability of fortified products. 6 It is estimated that 40% of the population is vitamin D deficient in the USA, 7 whereas among pregnant women, the prevalence fluctuates between 7 and 53.4%, depending on the country of residence. 8-12 The main causes of vitamin D deficiency are poor sunlight exposure, skin hyperpigmentation, obesity, cognitive impairment, institutionalization, reduced dietary intake, malabsorption and specific medications, such as anticonvulsants. 13
Lack of vitamin D results in poor intestinal absorption of calcium and phosphorus, with hypophosphatemia being an earlier electrolytic abnormality than hypocalcemia. Persistent vitamin D deficiency leads to secondary hyperparathyroidism, phosphaturia and bone demineralization causing rickets in children and osteomalacia in adults. 14 The association between reduced exposure to sunlight and rickets in children was first described by Sniadecki in 1822. 15 Since then, vitamin D deficiency has been associated with increased cancer risk, type 2 diabetes mellitus, adverse cardiovascular outcomes, and autoimmune diseases such as multiple sclerosis, type 1 diabetes mellitus and inflammatory bowel disease. 16-22
Considering the high prevalence of vitamin D deficiency and its unfavorable outcomes, the aim of this article is to provide an updated review on the effects of maternal vitamin D deficiency on the placento-fetal unit and the mother during pregnancy, and recommendations regarding supplementation.
Methods
We conducted a thorough literature search in the electronic databases of PubMed, Medline, Scopus, and The Cochrane Database of Systematic Reviews for literature available until March 2019. The following MeSH headings were used: “pregnancy” and “vitamin D deficiency”, in combination with “pre-eclampsia” or “gestational diabetes” or “preterm birth” or “small for gestational age” or “cesarean section” or “bacterial vaginosis”. Non-English studies and case reports were excluded. Moreover, in an effort to provide an updated perspective, we only focused on recent studies, published after 2007. The reference lists of retrieved studies were also scanned for other relevant ones. After applying exclusion criteria, 40 studies were deemed eligible and are discussed in this review.
Vitamin D deficiency and pregnancy outcomes
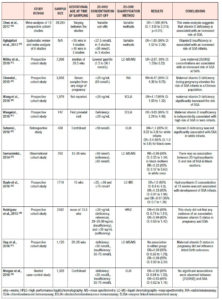
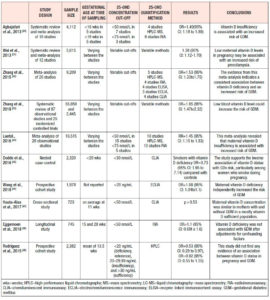
Pre-eclampsia (Table 1.)
Pre-eclampsia, defined as new-onset of hypertension and proteinuria in previously normotensive women after 20 weeks of gestation, affects 4.6% of pregnancies worldwide. 23
A meta-analysis, which included 9 studies, showed that pre-eclampsia was significantly related to decreased vitamin D levels, with a pooled OR of 1.79 (95% CI: 1.25 to 2.58),24 whereas in another meta-analysis, including 8 studies with 2273 participants, 25(OH)D levels were reported to be -3.86 lower in preeclamptic women than in controls (95% CI: -5.13 to -2.59).25 In line with these findings are several case-control studies, with varying sample sizes, which concluded that vitamin D deficiency is associated with pre-eclampsia in pregnancy – OR=3.24; 95% CI: 1.37 to 7.69, OR=2.23; 95% CI: 1.29 to 3.83, OR=2.18; 95% CI: 1.80 to 2.64 – and may increase the odds of pre-eclampsia up to 5 times.26-29 Neonates born to preeclamptic mothers had 2 times higher odds of vitamin D deficiency compared with neonates born to mothers without preeclampsia. 27,30
However, a few studies failed to show a correlation between vitamin D and pre-eclampsia.31,32 When vitamin D was measured in the first trimester, the development of pre-eclampsia was not significantly associated with low serum levels,33 although an increased trend was identified (OR=1.35 95% CI: 0.40-4.5).34 Two prospective studies, which did not find any correlation either, attributed the lack of association to the late gestational age at sampling, the small sample size, and the small number of women with pre‐eclampsia.35,36
Despite the inconsistent evidence among case-control and prospective studies, the underlying mechanism of hypovitaminosis D leading to pre-eclampsia has started to be elucidated. It is suggested that antiangiogenic factors, such as soluble FMS-like tyrosine kinase 1 (sFlt-1) and vascular endothelial growth factor (VEGF), are down-regulated by placental gene modification in in pregnant women with normal vitamin D levels . 37 Both factors, sFlt-1 and VEGF, have been associated with the development of pre-eclampsia in laboratory and clinical studies. 38-41
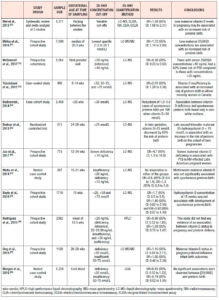
Gestational diabetes (Table 2.)
Vitamin D deficiency in the general population is associated with diabetes mellitus.18,21 Aghajafari et al. confirmed this finding among pregnant women in a meta-analysis of 9 studies without evidence of heterogeneity (P=0.58; I2=0.0%). 25(OH)D deficiency was associated with increased risk of developing gestational diabetes mellitus (GDM), with an OR of 1.49 (95% CI: 1.18 to 1.88), which increased further to 1.98 (95% CI: 1.23-3.23) when only studies with adjustment for critical covariates were considered.24
In agreement with the aforementioned study are most meta-analyses, which have reported OR values of 1.38 (95% CI: 1.12-1.70), 1.53 (95% CI: 1.33-1.75), 1.57 (95% CI 1.04-2.37), and 1.85 (95% CI: 1.47-2.33), whilst the association may differ in subgroup analyses based on countries, study design, assessment of vitamin D levels, sample size, age at baseline, adjusted models, study quality, and smoking status.25,42-45 In line with these findings, a Chinese cohort study showed that maternal vitamin D levels <20 ng/mL increased the risk of GDM, with an OR of 1.08 (95% CI: 1.04-1.1).46 On the contrary, there are observational studies that were not able to find any correlation between vitamin D status and GDM.47-49 However, it is suggested that HMW-adiponectin may be one of the mediators between vitamin D levels and adverse pregnancy outcomes, such as GDM.50
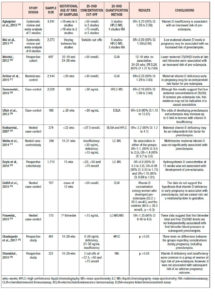
Preterm birth (Table 3.)
The evidence regarding hypovitaminosis D and preterm birth is less conclusive. Wei et al., in a meta-analysis of 5 studies, showed that vitamin D <50 nmol/L and preterm delivery were significantly correlated with an OR of 1.58 (95% CI: 1.08–2.31), without heterogeneity across the studies.25 A prospective study among 7,098 mothers in the Netherlands found that women in the lowest quartile of vitamin D levels, defined as 1.5 to 24.1 nmol/L, had an OR of 1.72 (95% CI: 1.14 to 2.60) for preterm birth, defined as <37 weeks of gestational age.51 Vitamin D concentrations ≥40 ng/mL seem to lower preterm birth risk by 60% among general obstetric patients.52 Interestingly, two studies showed greater association between vitamin D deficiency and preterm births among non-white/ethnic minority women than among white women.53,54
Both twin pregnancy and HIV infection are factors known to contribute to preterm delivery. Vitamin D-deficient women carrying twins had 60% increased risk of preterm delivery (<35 weeks) compared with women sufficient in vitamin D who also carried twins.55 The association between vitamin D status and preterm birth was found to be even higher among HIV-infected women, with an OR of 4.7 (95% CI: 1.3 to 16.8).56 On the contrary, other observational studies reported a lack of association between vitamin D status and preterm birth.31,32,49,57,58
Small for gestational age (SGA, Table 4.)
Less consistent is the evidence regarding maternal 25(OH)D deficiency and birthweight. A recent meta-analysis, which assessed 13 cohort studies with 28,285 individuals from seven countries, demonstrated an OR of 1.588 (95% CI: 1.138 to 2.216; p<0.01) for SGA infants among women with vitamin D deficiency.59 This inverse relationship between vitamin D status and birthweight was also found in two older meta-analyses; the first showed an OR=1.52 of SGA infants (95% CI: 1.08 to 2.15) for women with 25(OH)D levels <50 nmol/L,25 and the second an OR=1.85 (95% CI: 1.52 to 2.26) for women with vitamin D levels <75 nmol/L.24 Observational studies reported similar results for birthweight, namely OR=2.07 (95% CI: 1.33 to 3.22) when vitamin D levels were in the lowest quartile (1.5 to 24.1 nmol/L),51 and RR=6.47 (95% CI: 4.30 to 9.75)60 for vitamin D deficiency. Recent observational studies among Chinese women found that 25(OH)D <20 ng/ml increased significantly the risk of SGA infants, with OR values of 1.17 (95% CI: 1.03 to 1.32) and 3.05 (95% CI: 2.24 to 4.40) respectively. 46,61
Racial disparity has also been reported in the association between vitamin D status and birthweight, but the findings were contradictory; Seto et al. reported an association of vitamin D deficiency and SGA infants only among black women.62 On the contrary, other studies showed no association between vitamin D status and the risk of having a SGA baby.32,49,57,58 63
Other adverse pregnancy outcomes
Data regarding the impact of hypovitaminosis D on the delivery mode are inconclusive. It has been reported that vitamin D deficiency is associated with a fourfold increase in the risk of cesarean section.64 Less pronounced was the risk estimated by Scholl et al., who reported an OR of 1.66 (95% CI: 1.09 to 2.52).65 In contrast, other observational studies failed to show any correlation between serum vitamin D levels and delivery mode.35,66-68
Findings from three observational studies show an association between bacterial vaginosis in pregnancy and vitamin D deficiency. Using National Health and Nutrition Examination Survey data, Hensel et al. found that the adjusted OR of bacterial vaginosis was 2.87 (95% CI: 1.13 to 7.28) among gravidas with vitamin D deficiency (<30 ng/mL).69 A stronger association with an OR=4.4 was reported when a lower cut-off of 15 ng/mL was used to define vitamin D deficiency among pregnant African American adolescents.70 It is also concluded that women with normal vaginal flora have higher concentrations of serum 25(OH)D compared with women affected by bacterial vaginosis (geometric mean difference 10.6; p<0.001).71 It has been suggested that the increased risk of infection in pregnant women with vitamin D deficiency may be the result of high levels of proinflammatory cytokines, such as IL-6 and TNF-a.72
Vitamin D supplementation in pregnancy
A large body of evidence has assessed the need for vitamin D supplementation during pregnancy. In a meta-analysis of 9 studies, each of which used different doses of vitamin D, vitamin D supplementation was compared with no intervention or placebo. 73 It was found that vitamin D supplementation reduced the risk of pre-eclampsia, but the significance was borderline (8.9% vs 15.5%; RR=0.52; 95% CI: 0.25 to 1.05), whereas no protective effect was reported on the development of GDM. Furthermore, vitamin D supplements seem to exert a protective effect against preterm birth (RR=0.36; 95% CI: 0.14 to 0.93) and SGA infants (RR=0.40; 95% CI: 0.24 to 0.67), but no impact was shown on delivery mode.
According to the WHO, vitamin D supplementation is not recommended for pregnant women for maternal and perinatal outcome improvement, and women should simply be given advice regarding a balanced diet and sources of vitamin D. Nonetheless, if vitamin D deficiency is documented, the recommended nutrient intake (RNI) is 200 IU/day. 74
The Royal College of Obstetricians and Gynaecologists recommends that every pregnant and breastfeeding woman should be advised about vitamin D and prescribed 400 IU daily, based on the importance of vitamin D on calcium metabolism and on its non-calcium effects. For high-risk women -- namely with skin hyperpigmentation, reduced exposure to sunlight, or those who are socially excluded or obese --, the daily dose should be increased to at least 1000 IU/day. Moreover, 800 IU/day of vitamin D combined with calcium is suggested for women with a high risk of pre-eclampsia. Treatment doses of cholecalciferol 20,000 IU/week or ergocalciferol 10,000 IU twice/week for 4-6 weeks, followed by regular supplementation should be used for vitamin D deficient women. 75
As suggested by the American College of Obstetricians and Gynecologists Committee opinion, a standard prenatal dose of 400 IU of vitamin D daily should be prescribed. However, there is no sufficient evidence supporting vitamin D supplementation for preterm birth or pre-eclampsia prevention. Higher doses of 1,000-2,000 IU/day are recommended for pregnant women with vitamin D deficiency. 76
Conclusions
Vitamin D deficiency is highly prevalent worldwide, affecting people from different latitudes and regardless of their access to fortified products. Many observational, case-control and cross-sectional studies have demonstrated an association between maternal vitamin D status and adverse pregnancy outcomes, such as pre-eclampsia, gestational diabetes, preterm birth, small for gestational age, delivery mode and bacterial vaginosis; solid evidence, however, is still lacking. Variable cut-offs, different quantification methods, diversity between study groups, and confounding factors may account for the inconsistent results. Both the Royal College of Obstetricians and Gynaecologists and the American College of Obstetricians and Gynecologists recommend standard prenatal vitamin D supplementation of 400 IU, whereas WHO suggests a balanced diet instead of supplementation for pregnant women without documented vitamin D deficiency.