Introduction
In recent years, different definitions of polycystic ovary syndrome (PCOS) and guidelines for its management have been proposed. For both research and clinical purposes, the 2003 "Rotterdam criteria", based on the first workshop, are mostly used [1], with slight modifications as in the guidelines of the European Society of Endocrinology, [2] and those published in partnership by the European Society of Human Reproduction and Embryology (ESHRE) and the American Society of Reproductive Medicine (ASRM) [3,4]. All agree that PCOS is a common reproductive endocrine disease, which is characterized by hyperandrogenism, chronic anovulation, and polycystic ovaries.
The prevalence of PCOS in women of reproductive age ranges from 6% to 21% [5]. It accounts for about 75% of ovulatory dysfunction infertility [6], and seriously affects the fertility function of patients. Ovulation induction is the main treatment. To induce ovulation in patients with PCOS, the use of letrozole (LE) is now increasingly recommended. Indeed, in our department, treating every day about 200 patients with PCOS who want fertility, this treatment has often shown good results. However, sometimes it is not successful. The ovulation rate using LE is only 60%-80%, and other patients have no response [7]. Gonadotropin can be used in patients with first-line ovulation induction failure, but the incidence of multiple pregnancies and ovarian hyperstimulation syndrome is high [8].
Pulsatile gonadotropin-releasing hormone (GnRH) is a therapeutic approach for achieving induction of follicle growth according to the physiological regulation mechanism of the hypothalamus-pituitary-ovary (HPO) axis. To date, this treatment has mainly been used in idiopathic hypogonadotropic hypogonadism (IHH) and central secondary amenorrhea, achieving some curative effects [9]. Until now it has rarely been used in PCOS, although we use it in patients in whom we failed to achieve ovulation with LE. Here we report the case of an obese PCOS patient with spontaneous pregnancy induced by pulsatile GnRH after LE had failed to induce ovulation.
Case Report
In September 2018, a 31-year-old female of Han nationality visited our department with complaints of oligomenorrhea and infertility. She had a history of natural pregnancy and childbirth, without any diseases other than those reported herein. Menarche occurred at the age of 13 years, with regular cycles, 6-7 / 30 days. After delivery, her menstrual cycle extended to 30-90 days. Last menstrual period: August 15, 2019. Clinical examination revealed obesity (body mass index 29.9 kg/m2). There were no signs of hyperandrogenism. Pelvic examination did not show clitoromegaly. Her uterus was of normal size as assessed by transvaginal ultrasound, but polycystic morphology was seen in her ovaries: for each one, there were more than 12 follicles with a diameter of 2-9 mm.
Hormonal and other laboratory analyses gave the following results: follicle-stimulating hormone (FSH): 6.5 IU/L; luteinizing hormone (LH): 8.23 IU/L; estradiol (E2): 56.44 pg/ml; progesterone (P): 0.74 ng/ml; prolactin (PRL): 11.98 ng/ml; total testosterone (TT): 1174.73 pg/ml (normal range 90-550 pg/ml); free testosterone (FT): 14.9 pg/ml (normal range 0.8-7.4 pg/ml); anti-Müllerian hormone (AMH): 19.97 ng/ml (normal range 0.24-11.78 ng/ml); inhibin B (INHB): 16.21 pg/ml; high-density lipoprotein cholesterol (HDL-C): 0.85 mmol/L (normal range 1.04-1.6 mmol/L). The plasma levels of thyroid-stimulating hormone (TSH),fasting insulin (INS) and cortisol (COR) were within the normal range. In context with the anovulation and the polycystic ovaries, the diagnosis for this patient was PCOS.
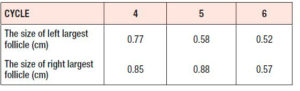
After 3 cycles of comprehensive pretreatment including nutritional guidance, weight loss and use of a combined oral contraceptive (Yaz: 20 µg ethinyl estradiol/3 mg drosperinone), she had been treated during six ovarian stimulation cycles with LE tablets (Jiangsu Hengrui, China) (LE), 5 mg per day for 5 days, from day 3 to day 7. In the first three cycles ovulation was achieved, but not in the next three cycles. Table 1 reports the maximum follicular sizes achieved during the last three cycles. On the second day of the menstrual cycle, the laboratory results were as follows: FSH: 8.08 IU/L, LH: 10.69 IU/L, E2: 302.94 pg/ml, TT: 84.29 ng/dl (normal range: 9-48 ng/dl), TSH: 0.88 mIU/L, uric acid (UA): 422.2 μmol/L, HDL-C: 0.94 mmol/L.
After informed consent of the patient, pulsatile GnRH was given on August 16, 2019 by subcutaneous pulse injection of gonadorelin analog (pulse every 90 minutes, 6 μg subcutaneous infusion each time). This treatment was officially performed within a clinical trial approved by the ethics committee of the Capital Medical University, Beijing, China (No.: 2019-qx-001-01).
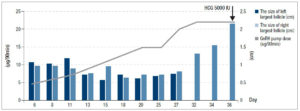
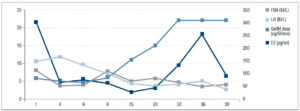
Follicular growth was monitored regularly every 2 to 3 days by B-ultrasound and the dose of pulsatile GnRH was adjusted gradually. Figure 1 shows the relationship between the dose of GnRH and FSH, LH, E2 levels; the relationship between the dose of GnRH and follicular size is shown in figure 2.
On the 33rd day of the menstrual cycle, the patient showed FSH: 4.66 IU/L, LH: 4.13 IU/L, and E2: 131.28 pg/ml, and the maximum follicle on the left side reached 1.31 × 1.01 cm. The dose of GnRH was adjusted to 22 μg/90 min and remained unchanged. On the 36th day of the treatment of pulsatile GnRH, the patient showed FSH: 3.42 IU/L, LH: 5.04 IU/L, E2: 254.89 pg/ml, TT: 48.57 ng/dl, and ultrasound revealed that the left ovary was 3.55 × 2.5 cm, right ovary was 3.55 × 2.5 cm, and the dominant follicle on the left had reached 2.17 × 1.91 cm.
We used human chorionic gonadotropin (HCG) 5000 IU to induce ovulation. Three days later, the patient showed FSH: 3.99 IU/L, LH: 2.77 IU/L, E2: 91.78 pg/ml, P: 2.03 ng/ml, PRL: 14.28 ng/ml, TT: 53.14 ng/ml - thus ovulation was achieved! On the 50th day of the menstrual cycle, the urine pregnancy test was positive. One week later, blood HCG was 1664.26 IU/L, P: 19.2 ng/ml. On October 28, B-ultrasound examination showed intrauterine pregnancy, i.e. fetal survival.
Discussion
Disruption of the HPO axis is considered one of the reasons for PCOS [10], which is mainly characterized by abnormal secretion of LH and FSH leading to disorder of ovarian follicle development and anovulation. Pulsatile GnRH therapy, in which GnRH analogs are injected subcutaneously, is used to simulate the physiological hypothalamic pulse secretion of GnRH, which can effectively stimulate the secretion of gonadotropins in the pituitary gland and then promote gonadal development, secretion of sex hormones and gametogenesis, and allow achievement of fertility [11]. At present, this medication rarely is applied in PCOS. Dubourdieu et al. [12] achieved good efficacy in the treatment of hypothalamic amenorrhea with PCOS by pulsatile GnRH with higher ovulation rate and pregnancy rate compared with combined gonadotropin treatment (FSH + LH). In this prospective randomized study, the dose of GnRH pulse was increased up to 20 μg/90 min, which achieved an ovulation rate of 73% and a pregnancy rate of 46%.
In 2015, Huiying Jia et al. [13] first applied pulsatile GnRH to treat a non-obese patient with PCOS and infertility in China; the patient became pregnant naturally and achieved delivery. However, the dosage in this study was not mentioned. Lu Lin et al. [14] combined this therapy with metformin using a pulse dose of only 10 µg/90 min; the treatment led to pregnancy with delivery in a non-obese PCOS infertility patient, further verifying the effectiveness of the treatment.
Already more than 30 years ago, HCG injection was used to trigger ovulation when the dominant follicle reached 18-20 mm, in order to more accurately predict ovulation and improve pregnancy rate [15]. This is in line with what we did in our study. Until now there have been almost no data in patients with PCOS. Dubourdieu et al. [12] used a single 250 IU recombinant HCG (rHCG) injection to trigger ovulation when one ovarian follicle reached 17 mm in diameter, but the remaining two cases were treated without HCG addition.
There is no uniform standard for the use and dosage of GnRH to induce ovulation in PCOS patients. In the studies mentioned above, the dose of GnRH varied according to the disease treated and the route of administration. Poor efficacy of GnRH observed in PCOS patients may be related to women’s different underlying endocrine characteristics. The pulse dose of GnRH should be adjusted gradually according to the endocrine and ultrasonic response of each subject during the treatment. The routes of administration include intravenous injection (IV) and subcutaneous injection (SC). For IV, it is reported that treatment of functional hypothalamic amenorrhea needed only 5 μg/90 min and IHH 7.5 μg/90 min, leading to ovulation rates of up to 100% per cycle. However, for PCOS, the ovulation rate was only 67.6% even using the high dosage of 20 μg /90 min [16]. For IHH, the recommended initial dose by IV is 2.5-5.0 μg per pulse. If this not effective after 10 days, the dose can be increased to 10 μg per pulse [17]. Treatment by SC usually requires a higher dose, the recommended initial dose being 15 μg per pulse [17]. If there is no dominant follicle with diameter greater than 10 mm on B-ultrasonic monitoring after a period of applications, the initial dose should be increased stepwise [17]. According to the consensus of experts on pulsatile GnRH therapy in China, 10 μg/90 min is recommended as the initial dose by SC [11]. Phlebitis is a complication that has been observed, particularly with IV application, according to the literature occurring with a rate of about 1.5% [18]. So, SC is safer and more convenient, and, clinically, it has become the best choice.
In our case, LE was prescribed to induce ovulation for six cycles. In the first three cycles ovulation was achieved, but not in the next three cycles. Because the woman wanted a second pregnancy, pulsatile GnRH therapy was initiated at the dose of 6 μg/90 min by SC, which was lower than the dose recommended in the literature described above. Follicular development was monitored dynamically every 2 to 3 days, starting from day 7, and the GnRH dose was adjusted according to the size of the follicle and the hormone levels. It ended up at 22 μg/90 min, which is similar to the ovulation dose used in Dubourdieu’s study [12]. After only one SC injection of pulsatile GnRH, given at day 36 of the treatment, the dominant follicle on the left grew to 2.17 × 1.91 cm. The bilateral ovaries were normal in size, so the risk of ovarian hyperstimulation syndrome was very low. We used HCG 5000 IU to induce ovulation, which can increase the chance of pregnancy. After this treatment, the LH, LH/FSH ratio, and testosterone levels decreased within one week and the woman got pregnant naturally within two months. All the treatments were performed without any significant side effects. So far, she has been regularly checked up for more than three months, and this follow-up has indicated that the pulsatile GnRH therapy was effective for her LE-resistant PCOS.
Conclusion
Pulsatile GnRH therapy can simulate the physiological pulse secretion mode of the hypothalamus, and this can restore the GnRH pulse secretion rhythm of hypothalamus in PCOS patients, leading to normal function of the HPO axis. This therapy can lead to ovulation followed by the chance of a successful pregnancy after failed ovulation induction using LE. To more accurately predict and induce ovulation and to improve pregnancy rate, HCG injection is recommended when the dominant follicle reaches 18-20 mm.
However, treatment with pulsatile GnRH is expensive, and the treatment process is complicated. It may cause local skin papule, induration and other adverse reactions, and this also limits its widespread application. Therefore, this treatment concept should be limited to special patients, such as PCOS patients resistant to other therapies, and it should be individualized and closely followed up in terms of doses and treatment schedule. Further studies including more patients should explore the best regimen, in terms of mode of application, timing and dosage, for using pulsatile GnRH in the treatment of PCOS cases. This would provide the basis for general recommendations regarding this treatment concept.
Acknowledgments
This study was supported by Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181401).
Disclosure statement
The authors declare no conflicts of interest.