Introduction
The incidence of cancer has been rising: one in nineteen women will have a neoplasm by the age of 49 (1). Survival rates have increased dramatically in recent years due to improvements in diagnosis and treatment. Death rates have fallen by more than 1.6% per year over the past five years. Chemotherapy and radiotherapy are common methods of cancer treatment and usually have permanent consequences. Infertility due to gonad damage is one of the worst consequences. Alkylating agents and pelvic radiation are more likely to cause gonadal dysfunction (2). A recent meta-analysis confirmed that radiotherapy was a risk factor for reproductive health problems in female childhood cancer survivors (3).
There are several techniques currently available to preserve fertility in cancer patients, giving them the opportunity to become mothers when they overcome the disease: cryopreservation of mature or immature oocytes followed by in vitro fertilization, cryopreservation of embryos, ovarian transposition and also cryopreservation of ovarian tissue. Combinations of different techniques could eventually be used (4,5).
Ovarian tissue cryopreservation is mainly indicated in cases where ovarian stimulation is not warranted, such as in pre-pubertal girls or in oncological patients (6-8). Research in ovarian tissue cryopreservation started in the ’50s and the first clinical studies in 2000. The procedure is performed in two stages (9). In the first stage ovarian tissue is surgically obtained either by laparoscopy or by laparotomy. The ovarian cortex is evaluated, frozen and cut into small pieces. Primordial follicles are located peripherally, which facilitates tissue cryopreservation. The cryopreservation can be performed by means of slow freezing or the vitrification technique. The vitrification process consists of ultra-rapid cooling at high concentrations of cryoprotectants, which avoids the formation of ice crystals (10). The tissue can be stored and in the second stage it can be autotransplanted (11). There is now enough evidence to support the technique and allow us to stop considering it an experimental and investigational approach (12).
As fertility preservation has been attracting growing interest among healthcare professionals, this study aims to review the current literature on ovarian tissue cryopreservation in cancer patients who went on to have successful live-birth pregnancies. We aim to evaluate different techniques that have been used by different research groups in oncological patients, hoping to elucidate and improve knowledge in this field.
Material and Methods
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The Medline and Cochrane databases were searched for reports published in English, Spanish and Portuguese between January 2004 and December 2019. The following Medical Subject Headings (MeSH) terms were used in different combinations: ovary; ovarian tissue; cryopreservation; transplantation and neoplasms. The initial date was chosen because it coincides the first publication on pregnancy after ovarian tissue cryopreservation autograft, by Donnez et al. (2004) (13). We complemented the search with a manual search of relevant articles, aiming to identify additional relevant studies not captured by our electronic search. Systematic reviews were consulted, but only data from original articles were included.
Eligibility criteria
Articles that reported ovarian tissue cryopreservation and clinical outcome of pregnancy with live births in oncological patients were eligible for analysis. Studies were selected for this review, using the following criteria:
- The study population comprised only women with cancer;
- The study population included women who were submitted to ovarian tissue cryopreservation and later autograft of this tissue after treatment for cancer;
- Final outcome of pregnancy with live-baby birth.
Two authors (J.M.S. and P.C.S.) independently conducted the selection of relevant studies. First, titles and abstracts were examined to determine whether the studies met the predefined selection criteria. After this step, the selected original articles were read for a final decision on whether or not to include them in the review. Any disagreements over the study selection between the investigators were resolved by consensus decision or by the decision of a third reviewer (D.Z.I.).
Results
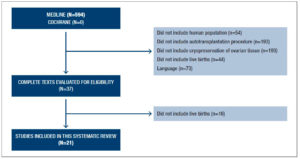
In total, 594 articles were identified (Figure 1). Careful reading of the titles and summaries and application of the inclusion criteria led to the exclusion of 573 manuscripts. Only original studies were included.
Among reports of more than 100 pregnancies obtained through the technique in all patients, we found 57 reports of successful pregnancies in cancer patients who had undergone autotransplantation of cryopreserved ovarian tissue. It is important to stress that only published live births could be included in this analysis. A review paper published in 2014 reports around 35 live births after cryopreservation of ovarian tissue in cancer patients, however, this article was based on phone calls made by Dr. Silver to his colleagues instead of published papers (14).
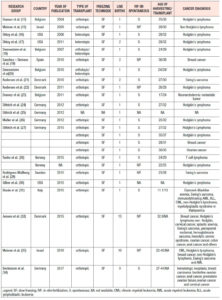
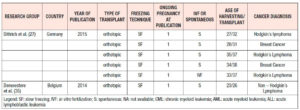
The first pregnancy after cryopreserved ovarian tissue autografting was published by Donnez et al. in 2004 (13). Table 1 shows the data of the successful pregnancies reported in the literature (13,15-34). Table 2 shows data referring to some of the ongoing pregnancies published in the literature that were not taken into account (27,35)
The participants
Among all the women, 78% had hematological malignancies, 15% had breast cancer, 13% Ewing’s sarcoma, 5% neuroectodermal metastatic tumor, 5% brain cancer, and 6% ovarian cancer as their primary diagnosis.
The mean age of the women at the time of ovarian tissue cryopreservation was 28.2 years, ranging from 17 to 45 years; at the time of reimplantation of the graft, their mean age was 31.2 years, ranging from 24 to 38 years.
The technique
In the majority of the cases (i.e. all except one), the harvest was performed laparoscopically. Also, in the majority of live-birth cases the pregnancy was obtained through the slow freezing technique, except for one case in the United States.
This review includes one report of heterotopic transplantation. The woman had a remaining ovary that explains her pregnancy, because no follicles were viewed in the follow up of the graft tissue. There is one report in the literature that describes a clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman with previous bilateral oophorectomy. This case is considered the first pregnacy by a heteropic graft (36); however this report was not included in our review because it was published as an ongoing pregnancy. Finally, in 75% of the cases spontaneous pregnancy occurred after autotransplantation and in 25% in vitro fertilization techniques were used.
Discussion
The studies in the present review show that numerous centers of human reproduction around the world are performing ovarian tissue cryopreservation; however, few of them have actually performed ovarian tissue reimplantation. Also, the negative results of some centers may not be readily published and may be difficult to find. This makes it difficult to calculate the real success rate of the procedure. In a review published by Donnez et al. (12), a reimplantation success rate of 25% was reported. The mean duration of ovarian function after transplantation was 4-5 years.
The patients selected for autotransplantation were mostly women who had lymphoma or breast cancer, in whom the technique has been shown to be safe and is in accordance with the existing literature.
In our sample, laparoscopy was used in the majority of the cases due to its benefits. It is indeed possible to affirm that the laparoscopic technique for collecting ovarian tissue is the most appropriate, because it is a less invasive procedure than laparotomy and is therefore more beneficial to the patient.
The majority of the reports used the slow freezing technique, but research using vitrification in animals and humans has given good results. In fact, a study conducted in animals in our service showed oocyte recovery and survival after warming of a vitrified oocyte (37). A study published in 2015 reported successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency (38). The vitrification process prevents the formation of ice crystals and preserves a higher amount of ovarian stromal tissue (10). Another study published in 2015 studied vitrification and vascular bed in monkeys and it was found that the vitrification technique associated with a freshly decorticated bed is the best combination for obtaining functional autografted ovarian tissue (39).
In most cases an orthotopic transplantation technique was used, even though positive outcomes have been reported with the heterotopic technique. Oktay et al. stressed that the “occurrence of spontaneous pregnancies after heterotopic ovarian transplantation highlights the need for caution when interpreting the source of pregnancies in recipients with intact ovaries” (16). Heterotopic transplantation is interesting because surgery is less invasive and monitoring is easier, however, more studies are necessary before this technique is adopted. To date there is only one confirmed case of clinical pregnancy after heterotopic reimplantation of cryopreserved ovarian tissue (36).
Research is being performed to improve the cryopreservation technique and to demonstrate the possibility of in vitro maturation of ovarian follicles. This appears to be a promising technological advance since it could eliminate the major concern of the literature, which is the possible reimplantation of malignant cells into the patient at a time when the disease is already in remission.
Another target in the research field is to enhance the vascular bed before reimplantation to increase pregnancy rates. It is known that more than 50% of primordial follicles are lost after autografting, mainly due to tissue ischemia after transplantation while awaiting angiogenesis. Reoxygenation of ovarian grafts occurs from day 5 onward, and this affects the longevity of the graft (10). If neoangiogenesis could be enhanced, the effectiveness of the technique would probably be greater (40,41).
All the existing technology and the promising advances in oncofertility show us that young cancer patients with chances of survival should not be deprived of the chance to preserve their fertility before gonadotoxic chemotherapy. The options should be discussed individually to increase the chance of these patients becoming mothers in the future.
Conclusions
Cryopreservation of ovarian tissue followed by autotransplantation is a consolidated technique in fertility preservation (42). Female cancer patients of reproductive age benefit from this approach, given that gonadotoxic chemotherapy treatment for their disease often cannot be delayed. In the literature we found 57 cases of women diagnosed with cancer who had successful pregnancies with live births after they were considered free of the disease. In the majority of women (i.e. except for one), ovarian tissue was cryopreserved by a slow freezing technique, and all except for one had orthotopic transplantation of their own ovarian tissue into the pelvic cavity. The success rate of this technique is hard to calculate because many centers worldwide do not report their negative results, however Donnez´s study group published a reimplantation success rate of 25%. There is concern over the possibility of reintroducing malignancy with the technique, but it has been shown that this procedure is considered low risk in cases of lymphoma, cervical cancer and early stages of breast cancer. In vitro maturation of oocytes is a near future development that may enhance this technique and research on improving the vascular bed of the autograft is promising.