Introduction
Since inception in 1942, hormone therapy (HT) has benefited from a personalized approach through physician/patient interactions to arrive at an optimal formulation and dose to treat menopausal symptoms. This very personalized approach has generated decades of data that can be combined with 21st century genomic technologies to create a precision medicine approach to HT. Personalized clinical care powered by precision medicine analytics has the potential to advance HT to optimize both efficacy and safety 1,2.
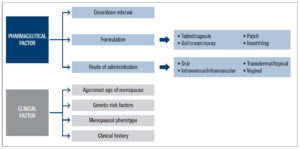
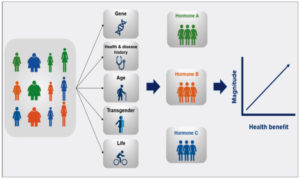
Precision medicine is best exemplified by selection of cancer treatments which has pioneered personalized precision medicine by combining the genetics of cancer patients with genomic technologies and big data acquired from millions of patient records to screen treatments against the patient’s own tumor cells. In contrast, HT for menopausal symptoms poises a much more complex challenge as HT is a whole body/multi-organ intervention that requires consideration of patient-specific characteristics including genetic variability, physiological health, age, gender, environment, and lifestyle (Figure 1) 2,3. HTs approved by the U.S. Food and Drug Administration (FDA), the Health Products and Food Branch (HPFB) of Health Canada, and the European Medicines Agency (EMA) are well characterized thereby providing a well characterized set of therapeutic interventions. Big data and computational systems biology analytics can be utilized to analyze medical records to detect patterns and associations between variables listed above and the short and long-term health outcomes associated with specific HTs.
In considering precision HT, we first provide a historical perspective followed by an analysis of the current state of the field concluding with near term opportunities to advance development of precision HT.
Historical Perspective on Drivers of Precision Hormone Therapy:
HT was first introduced in the 1940s with transition to greater use in the late 1960s 4. The use of HT increased again after 1988 when the FDA approved estrogen therapy to prevent postmenopausal osteoporosis 4. Based on a national pharmacy claims database, the prevalence of women using HT was 21.6, 21.4, and 20.9 % in 2000, 2001, and 2002 respectively, among women aged 50 years and older 5. Observational cohort studies of HT users suggested decreased mortality of coronary heart disease by 20-40% and reduced risk of Alzheimer’s disease 4.
HT has consistently reduced menopausal symptoms whereas the association with longer term health outcomes has illuminated the urgent need of a precision medicine approach to HT. As the number of women using HT use increased, adverse effects became more apparent in the mid 1970s 4. Data emerged indicating increased risks of breast cancer, uterine cancer, and stroke in estrogen therapy users 4. Thereafter, a progestogen was combined with estrogen therapy to prevent uterine cancer. However, studies suggested a decrease in the attenuating effect of HT on cardiovascular disease when estrogen therapy was combined with a progestogen 4.
Increased concern regarding safety of HT drew the attention of the National Institute on Health (NIH) and the FDA which identified a need for large randomized clinical trials of HT 4. The Women’s Health Initiative (WHI) was one of the outcomes of those concerns. The WHI clinical trials recruited 16,608 healthy postmenopausal women aged 50 to 79 years with an intact uterus at baseline in 1993-1998 for HT trials, and participants received 0.625 mg of oral conjugated equine estrogens (CEE) with 2.5 mg daily medroxyprogesterone acetate (MPA) or placebo 6,7. The HT trials also included 10,739 women who were post-hysterectomy at baseline and received either unopposed estrogen therapy consisting of 0.625 mg of CEE daily or placebo 7. The initial purpose of the clinical trials was to determine whether HT prevented from heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women 8.
A turning point in HT use in women occurred following the report of WHI outcomes in 20028. Initial findings from the WHI study were unexpectedly negative, indicating that overall health risks exceeded benefits in the combined CEE and MPA group who had on average 5.2-years of follow-up. Outcomes of these analyses were that the combination of CEE and MPA should not be initiated or continued for the primary prevention of coronary heart disease as well as identified risks of cardiovascular disease and breast cancer 6,9. These findings derived from a single HT formulation and dosage were generalized to all formulations and doses.
Following the initial report, additional studies were conducted to investigate the association of HT with multiple outcomes including venous thromboembolic (VTE) events 10,11, cognitive function 12-18, stroke 19, and age-related diseases 20,21. A lower oral dose (0.3 mg) of CEE reduced the risk of stroke relative to the higher dose (0.625 mg) 22. Increased risks of dementia and cognitive impairment during and after HT were closely associated with occurrence of type 2 diabetes 23. HT was beneficial for women who are at risk of osteoporotic fractures and low bone mineral density 6. Overall, the impact of the WHI has been sustained and remains a factor in considering HT by women and their physicians.
Factors Influencing Precision Hormone Therapy
Age and response to HT
The impact of age on HT response is illustrated in two studies. In a randomized longitudinal study of postmenopausal women with a mean age of 57.7 year, impact of HT on an indicator of aging, telomere length, was investigated 24. Outcomes of these analyses on a biomarker of aging biology indicated that APOE-e4 carriers had marked telomere attrition during the 2-year study window which was equivalent to approximately one decade of additional aging compared to non-carriers. Further analyses revealed a modulatory effect of HT on the association between APOE status and telomere attrition. APOE-e4 carriers who continued HT during the 2 year trial sustained telomere length and did not exhibit signs of aging, whereas women who discontinued HT telomere length shortened consistent with accelerated aging 24. Women who did not carry the APOE4 allele exhibited no protective effect of HT on telomere length 24. The impact of HT in APOE4 positive women in the Nurses’ Health Study indicated that cognitive function in 70–81 year old APOE4 carriers currently using HT was associated with a slight increase in rate of decline 25. Transdermal estrogen therapy was advantageous for women at risk of VTE, as the first pass metabolism of oral estrogen-only HT increases resistance to activated protein C, which is a natural anticoagulant 26.
Although benefits of HT in symptomatic peri to menopausal women were well documented, the negative outcomes from WHI in postmenopausal women resulted in a dramatic decrease in HT use from 21.6% in 2000 to 8.8% in 2009 in women aged 50 years and older 5. Prescription claims for US commercial health insurance indicated that the age-standardized annual prevalence of oral estrogen prescriptions was 83 per 1,000 women in 2007, and decreased to 42 per 1,000 women in 2015 27. The majority of the WHI-Memory study population was older than 60 years with only 20% of enrolled women aged 50-60 years and less than 5% were 54 years of age 4. Thus, it is important to note that WHI-Memory study findings were based on ~ 75% of women who were 10+ years post menopause and thus quite different from age of women (45-55 years of age) for whom HT is typically prescribed. Despite the advanced age of women receiving HT, the WHI findings resulted in the discontinuation of HT use in all age groups including younger postmenopausal women 28.
To assess differences in HT use before and after the WHI, Crawford et al. 28 analyzed survey data collected from 3,018 midlife women in a prospective cohort study conducted between 1996 and 2013 from the Study of Women’s Health Across the Nation. They divided participants into four age groups: 42-49.5 years, 49.6-53 years, 53.1-57.1 years, and 57.2 years and older. Overall, HT initiation decreased from 8.6% (pre-WHI) to 2.8% (post-WHI). Based on their analysis, the percent of HT initiation was the highest in 49.6 and 57.1 years age groups before the WHI, but were the age groups with largest decrease after the WHI 28.
Currently, FDA advises women to use HT for the shortest time (generally less than five years or not beyond age 60 years) and at the lowest dose possible to treat menopausal symptoms 29,30. Collectively, the data indicate that age, a critical variable for precision HT31, significantly impacted response to HT. Thus, extrapolation of outcomes of HT initiated in older women, such as in the WHI, to younger perimenopause to early menopausal women, who may benefit from HT, is problematic as multiple molecular pathways in multiple organ systems change across the menopausal transition31-33.
Hormone therapies approved by regulatory agencies
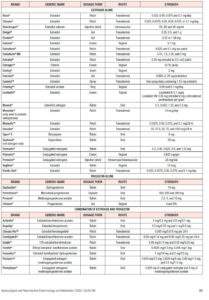
There are 39 HT products approved by regulatory agencies in the U.S. (FDA), Canada (HPFB), and Europe (EMA) composed of 13 potential estrogen- or progestogen-types of steroids, over 12 different dosage forms, and administered via 4 routes of administration, resulting in multiple pharmacokinetic profiles (Table 1).
Impact of progestins
Different types of progestogens have been utilized in combined HT with estrogen, which include progesterone naturally secreted by ovary and placenta, and progestins (synthetic forms of progesterone) such as MPA, dydrogesterone, norethindrone/norethisterone, norethisterone acetate, and levonorgestrel 34. It has been reported that the type of progestogens in HT is one of the critical factors to be optimized, as they have shown different effects on risks associated with HT, such as breast cancer and cardiovascular diseases. Previous studies demonstrated a relative low risk of breast cancer 35,36 and VTE 37 by using dydrogesterone. On the contrary, a relative high risk of VTE was observed among MPA users as compared to norethisterone/norgestrel users 11.
One of the potential reasons for different effects of progestogens on the risk of breast cancer risk may be explained by their different binding affinity to steroid receptors expressed in breast cancer cells, including estrogen receptor (ER), androgen receptor (AR), glucocorticoid receptor (GR), mineralocorticoid receptor (MR), and progesterone receptor (PR)38. In addition, different biological activities of metabolites of progesterone and progestins may contribute to proliferation or apoptosis of breast cancer cells. An example are the opposing actions of two different progesterone metabolites, 5a-dihydroprogesterone and 3a-dihydroprogesterone, on mitosis, apoptosis, and expressions of B-cell lymphoma 2 (Bcl-2), Bax (an effector of apoptosis in breast cancer), and p21 (a potent cyclin-dependent kinase inhibitor) in human breast cell lines 39.
Different progestogens exert different cellular and neurobiological outcomes 40-41. MPA exacerbates glutamate-induced neurotoxicity in primary hippocampal neural cultures, regardless of its formulation types and chemical structures (crystalline MPA versus a pharmaceutical formulation, Depo-Provera®) whereas progesterone promoted neuroprotection 40. The impact of progesterone and MPA on glycolysis, oxidative stress, and mitochondrial function in neural tissue was progestin specific 41. In contrast to progesterone, MPA diminished glycolytic and oxidative phosphorylation protein and activity, and reduced estradiol-induced enhancement of mitochondrial respiration in hippocampal neurons and glia, which eventually exacerbate oxidative damage and neurodegeneration 41.
Alternatives to progestins
As an alternative to progestogen therapy for women with an intact uterus, Duavee® (approved by the FDA in 2013) is a combination product containing conjugated estrogens and bazedoxifene which is a selective estrogen receptor modulator used for the prevention of osteoporosis. The efficacy and safety of this combination was evaluated in five phase 3 Selective estrogens, Menopause, And Response to Therapy (SMART) trials 42. Further, bazedoxifene was reported to inhibit the proliferation of endocrine-resistant breast cancer cells 43. The combination of conjugated estrogens plus bazedoxifene reduced vasomotor symptoms and osteoporosis-related fractures, increased vulvar/vaginal atrophy without increasing indicators of cardiovascular disease or endometrial and breast cancer in postmenopausal women in the SMART studies 44-49.
Treatment Regimen
Treatment regimen (cyclic versus continuous) of combined HT has been addressed as one of the factors impacting HT-associated breast cancer risk 50 . Cyclic HT with the combinations of 17β-estradiol and progesterone is suggested to be more effective than their continuous use, because a cyclic exposure to progesterone closely mimics the natural female hormone pattern, inducing gene expression profiles to be consistent with the ovary intact brain 50. Another study presented that cyclic progesterone reduced b-amyloid levels and enhanced 17b-estradiol effects in a transgenic mouse model of Alzheimer’s disease 51. It has been also suggested that progesterone administration in cyclic regimens does not affect a risk of breast cancer 52. Cyclic or continuous MPA with estrogen inhibited vasodilatation by 50%, however, there was no diminished estrogen-induced vasodilatation by nomegestrol acetate 53.
Non-hormone therapies for menopausal symptoms
In addition to hormone-based therapies, there are two FDA-approved non-hormone drugs to treat menopausal symptoms : (1) BrisdelleTM (approved in 2013), an oral capsule form of paroxetine, a selective serotonin reuptake inhibitor indicated for the treatment of moderate to severe vasomotor symptoms due to menopause 54 and (2) Osphena® (approved in 2013), an oral tablet of ospemifene, an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia and vulvar and vaginal atrophy due to menopause 55.
Bioidentical and compounded HTs
Patient and physician interest in bioidentical and compounded medicines has grown as an alternative option of FDA-approved hormone medicines. The bioidentical hormone drug contains active ingredient(s) with the same chemical structure of human hormones. In 2018, the FDA approved the first bio-identical oral hormone combination of 17β-estrogen and progesterone, Bijuva, indicated for women with a uterus for the treatment of moderate to severe vasomotor symptoms associated with menopause56.
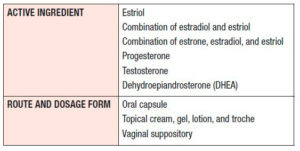
Alternatively, compounded hormone formulations are not regulated by the FDA and are generated by compound pharmacies that often, but not consistently, contain United States Pharmacopeia (USP)-grade steroids 57. Compounded HT is formulated as capsules, creams, gels, lotions, trochs, and suppositories (Table 2) 57,58. These formulations are not approved nor regulated by the FDA 57. Further, the FDA does not regulate production processes, number of users nor track adverse outcomes. Although there is a lack of verification regarding safety of compounded steroid therapies, women select compounded hormone drugs because of increased public concern regarding risk factors of the current FDA-approved HTs and unawareness that compounded hormone drugs are not approved by the FDA 59,60.
Because the number of compounded drug users is not officially tracked, surveys serve as a proxy to characterize the demographics of the compounded drug users. The North American Menopause Society (NAMS) conducted an Internet-based consumer survey in 2015. An age of participants in the survey was between 40 and 84 years. Among 3,725 women enrolled and eligible for the survey, 28% of the women were current or past HT users, and 31% used compounded hormone drugs and the majority (69%) were FDA-approved hormone medicine users. One of the interesting observations in this survey was that the percent of compounded hormone users was highest (41%) in early age group (40-49 years) and the compounded hormone users tended to be younger than the FDA-approved hormone users. The percent of women who selected FDA-approved HT was higher than the compounded users in all age groups, but the percent was the highest in the oldest age group. Furthermore, almost one-third of the women were not sure whether the HTs they used was approved by the FDA 59. Another large internet survey conducted by Harris Interactive Inc also reported that 86% of women surveyed (aged 45-60 years) were unaware that compounded drugs were not approved by the FDA 60. These survey results stress two important gaps: (1) the need for public education regarding the use of compounded hormone drugs, and (2) the necessity for a verification process for compounded hormone drugs in terms of safety and efficacy. With growing interest in compounded hormone drugs, the FDA announced an agreement with the National Academics of Science, Engineering & Medicine (NASEM) for two studies to examine the clinical utility of patients treated with compounded hormone products, and the safety and effectiveness of multi-ingredient compounded topical pain creams 61.
Due to uncertainties regarding safety, the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and the Practice Committee of the American Society for Reproductive Medicine cautioned against the use of compounded hormone formulations 62.
Advancing Precision Hormone Therapy Opportunities
Genetic factors influencing clinical use of HT:
Genetics can determine response to HT and provide a technologically feasible strategy to achieve precision HT. One well described impact of formulations relevant to precision HT is the induction of venous thromboembolism (VTE) in women carrying the Factor V Leiden (rs6025) gene variant with oral estrogen therapy 63,64. In non-carrier HT users, the relative risk for VTE is increased 2- to 4-fold relative to non-users of HRT. In contrast, HT users with Factor V Leiden genetic variant showed an increased risk for VTE between 7- to 15-fold relative to non-carriers and nonusers 64,65.
Hepatic first-pass metabolism that occurs with oral administration of estrogen induces liver clotting factors leading to VTE, which can be avoided by transdermal administration 63,65. The study by Laliberté et al. 26 showed a comparison of the risk of VTE between oral- and transdermal-estradiol administration. They conducted a claims analysis using the Thomson Reuters MarketScan database from January 2002 to October 2009, which included participants aged 35 years or older and newly used transdermal or oral estrogen-alone therapy. Each group included 27,018 women, and 115 transdermal-estradiol users developed VTE, which was a significantly lower incidence compared to oral-estradiol users (164 women) 26. The benefit of transdermal administration for estradiol therapy was associated with the avoidance of hepatic first-pass metabolism of estradiol. Orally administered estradiol undergoes first-pass metabolism, and it has been known that estrone, a major metabolite of estradiol, can affect thrombin generation, a marker of hypercoagulability which is used to determine thrombotic risk associated with HT 10,11,66,67. Thus, a transdermal route has been suggested as a safe option for women at a high risk of VTE compared to an oral route.
In addition, biomarkers relevant to cardiovascular disease was proposed by Manson 1, which includes lipids (e.g. serum LDL cholesterol, LDL/HDL ratios, triglyceride, 27-OH-cholesterol, and apolipoprotein), inflammatory markers (e.g. high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor α, and leukocyte count), adipokines (e.g. adiponectin, leptin, and retinol binding protein-4), endothelial marker (e.g. E-selectin, P-selectin, ICAM, and VCAM), glucose tolerance markers (e.g. fasting glucose, insulin, HOMA-IR, and IGF-1), matrix metalloproteinases, hemostatic markers (e.g. D-dimer, factor VIII, von Willebrand factor, homocysteine, fibrinogen, and tissue factor pathway inhibitor or acquired activated protein C resistance), sex steroid hormone level, and sex hormone binding globulin level 1.
Genetics associated with breast cancer are a well documented consideration of HT use. To reduce the risks of breast and ovarian cancers, bilateral prophylactic oophorectomy is selected among BRCA1/2 mutation carriers 68. In particular, BRCA1/2 mutations play a critical role in increased breast and ovarian cancer risks69-71. However, HT is prescribed for women suffering from menopausal symptoms after surgery 72. There has been discussion whether HT mitigates the protective effect of bilateral prophylactic oophorectomy on decreased risks for breast or ovarian cancer. However, studies have demonstrated that short-term use of HT does not reduce the protective effect of oophorectomy surgery in breast cancer patients 69. Furthermore, the use of HT was reported to decrease breast cancer risk in postmenopausal women with a BRCA1 mutation 70. A recent study suggested that a combined treatment of mifepristone, a selective progesterone receptor modulator, and progesterone exerted anti-proliferative effect on ovarian mesenchymal stem/stromal cells of healthy female BRCA1/2 carriers in vitro 73.
In addition to BRCA 1/2 mutations, common markers associated with breast cancer tumors are the estrogen receptor (ER), the progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) 74. In addition to these receptors, a host of other genes associated with increased risk for breast or ovarian cancer includes: (1) PALB2, ATM, CHEK2, and MSH6 for a risk for breast cancer, and (2) MSH6, RAD51C, TP53, and ATM for a risk for ovarian cancer 71.
Cytochrome P450 (CYP) enzymes are involved in metabolic conversion of estrogens, which are relevant to individual’s response to drugs 75. Estradiol and estrone are metabolized by irreversible hydroxylation catalyzed by the NADPH-dependent CYP enzymes. Genetic polymorphisms in genes encoding CYP1A1, CYP1B1, CYP17A1, and CYP19A1 can impact estrogen metabolism and therefore impact downstream consequences 75. Risk of breast cancer was significantly affected by genetic polymorphisms in CYP1B1 76.
Clinical conditions impacting response to HT:
Estrogen plays a critical role in the bioenergetic system of the brain and promotes glucose transport, aerobic glycolysis, and mitochondrial function 33,77-79. In addition to regulating expression of glucose transporters, estrogen promotes the insulin-sensitive glucose transporter 33. Type 2 diabetes is a risk factor for dementia and in particular Alzheimer’s disease 78. Espeland and colleagues investigated the effect of HT on brain volumes and incidence of cognitive impairment in postmenopausal women based on type 2 diabetes status 79. Outcomes of their analyses indicated that increased risk of cognitive impairment occurred among menopausal women with type 2 diabetes or emerging diabetes which was paralleled by changes in gray matter (total and hippocampal) volume 79,80.
Conclusion
From a clinical perspective, precision HT is critical to delivering personalized medicine for management of menopausal symptoms and bridging the gap between health span and life span in postmenopausal women. Achieving precision HT requires inclusion of multiple factors including age, genetic risk factors, symptomatic phenotype and clinical history (Figure 2). From a pharmaceutical perspective, determining the optimal HT depends on the molecular constituents, dose, treatment regimen, formulation and route of administration. Much of the data required to achieve precision HT is available but with varying degrees of accessibility. Barriers to achieving precision HT can be overcome by increasing access to electronic medical records for medical informatic and computational systems biology analysis. Outcomes of these analyses could form the foundation of an algorithm decision making tree 30 which incorporates genetic risk factors, patient clinical data with, pharmaceutical data on different HTs to optimize HT. Remarkably, the patient and physician goal of personalized HT aligns with the currently available data and analytic technologies to achieve precision HT. Development of precision HT will significantly advance womens’ health while also creating a foundation on which to advance precision contraceptive medicine and male HT.
Acknowledgement
This work was supported by NIH grants R37AG053589, 1R01AG057931 and P01-AG026572 to RDB.