Introduction
Hypoactive sexual desire disorder (HSDD) and difficulty in reaching orgasm are two of the most frequent female sexual complaints1-3. Although these symptoms worsen in postmenopause, a period in which the androgen / estrogen ratio inverts due to hypoestrogenism, there is a high prevalence of decreased libido in women in premenopause too, a phase in which there are still high levels of circulating estrogen. Therefore, the role of testosterone and its precursors in the development of these symptoms must be considered4-7.
HSDD is characterized by absent or reduced motivation to engage in sexual activity or fantasies for at least 6 months, leading to personal suffering. It can be primary or secondary, generalized or specific, and it affects about 10% of women of reproductive age in the USA2,8,9. Non-sexual mental disorder, anguish in the relationship, and effects of a substance or medical condition must be ruled out as other possible causes of lack of desire8,9.
The association of HSDD with testosterone is not clear, mainly because biochemical tests do not show good sensitivity to low values, as found in female serum, due to hormonal variation during the menstrual cycle10-15.
Even though several studies have shown that transdermal testosterone, associated or not with estrogen therapy, is one of the possible treatments for decreased sexual desire in postmenopause10,11, there is still no consensus on its effective dose, side effects, or on whether it has positive results in terms of increasing libido in premenopause16-24. Some authors have also demonstrated a relationship between androgen therapy and behavioral changes including aggression, irrationality, mood and unstable personality20.
So far, it is known that testosterone does not determine aggression or changes in sexuality per se, and that, in parallel, it has been more associated with dominance than aggressiveness itself25-30. However, due to the presence of conflicting and limited data, this study seeks to better understand the role of testosterone in women with HSDD in premenopause, both in the genesis and treatment of this condition, and as regards a possible correlation with tendency-to-aggressiveness behavioral changes.
Methods
The trial was a randomized, double-blinded, placebo-controlled study performed at the gynecological endocrinology clinic of the Paulista Medical School in São Paulo, Brazil. It was conducted in accordance with the ethical principles of the Declaration of Helsinki and the protocol was approved by the university ethics committee. The study is registered with Ensaiosclinicos.gov.br (RBR-27CQN5).
The primary objective of the study was to describe possible correlations between tendency to aggressive behavior and enhanced sexual desire in patients diagnosed with HSSD and treated with a nanostructured transdermal testosterone gel (daily dose of 300 mcg). The secondary objective was to look for associations between the use of testosterone and improved sexual desire, behavioral changes (i.e. a tendency to aggressiveness), and increased serum levels of free and total testosterone.
Patients
It was established that to detect, within a group, a 1 percentage point increase or decrease in mean EATA (Scale to Assess Tendency to Aggressiveness) and mean FSFI (Female Sexual Function Index) scores with a power of 90% and an α of 95%, a sample size of 24 participants per group was required. All data were processed using the STATA program (version 7).
Patients attending the gynecological endocrinology clinic of the Paulista Medical School, diagnosed with HSDD, aged between 18 and 45 years, with physiological menstrual cycles, steady relationships, and using contraceptive methods were screened, given a physical examination, and invited to participate in the research and sign the informed consent term. Participants with a BMI below 18 kg / m2 or above 35 kg / m2, hyperandrogenic syndrome, severe clinical illness or severe depression, pregnant women, lactating women or women with a history of androgen therapy in the last 6 months were excluded from the study. Patients over 40 years old should have had a normal mammogram within the last 2 years.
Study procedures
The participants who met the selection criteria were divided by the researcher, using the Random.org program, into two groups according to a 1: 1 ratio to receive formulation A or B, provided by Evidence Soluções Farmacêuticas Ltda. The treatment consisted of one dose daily for a period of 12 weeks applied to the inner arm or thigh. The participants and the researchers remained blind until the end of the analysis of the results, when Group A was revealed as placebo with Biolipid/B2® vehicle and Group B as testosterone 300 mcg with Biolipid/B2® vehicle after breaking the code (double-blind). As there is no standard treatment for HSDD, the patients in the placebo group were not undertreated.
Biolipid/B2® is a transdermal nanostructured drug delivery system produced exclusively by Evidence Soluções Farmacêuticas Ltda with a US patent (2013/0123221 A1). Composed of esters of cholesterol and free fatty acids, it forms a micellar structure, homogeneous and stable when in contact with the skin, and according to the skin permeation evaluation by spectroscopy, the formulation Biolipid/B2® with testosterone is available in the microcirculation within the first hour after use and remains subsequently 24 hours evaluation29.
Symptomatic events were analyzed at the beginning and at the end of the treatment through anamnesis, physical exam and evaluation of scores obtained on the two questionnaires: the FSFI (cutoff value 26, less than that indicates a risk of developing sexual dysfunction), and EATA (cutoff value 17, more than that means a high tendency to aggressiveness), plus evaluation of laboratory samples of free testosterone, total testosterone and sex hormone-binding globulin (SHBG) sent to a partner laboratory chosen for the study (Posenato Laboratory).
At the end of the research it was expected that patients would have recovered their libido with low or no side effects. Participants who did not have increased sexual desire were invited to attend a specialized clinic to define a treatment, not necessarily with testosterone.
Statistical methods
Data were collected through questionnaires; in addition, continuous and discrete variables were collected during the screening and follow-up exams. Data were tabulated in the Excel program (Microsoft Windows 2010). The random distribution program was Random.org.
The codes in the “code book” remained hidden until the data had been fully analyzed. All study participants, including outcome assessors, remained blind until the end of the analysis.
The statistical analysis was performed using the R program, latest version 3.6.1. Descriptive analyses of all variables of interest for the study were carried out. Numerical or continuous variables were summarized based on measures of central tendency and dispersion, in order to determine the appropriate statistical tests (parametric or non-parametric), and tests were performed to measure normality and estimate asymmetry measures.
The relationship between age and each of the variables related to the study objective was analyzed, mainly with the scores obtained in the questionnaires evaluated through correlation analysis, Student's T test or Mann-Whitney U test, taking into account the sample distribution in two groups. In the same way, Pearson's Chi Square test was performed for contingency tables according to the characteristics of the other study variables (sociodemographic data, FSFI and EATA scale scores, as well as the testosterone and SHBG values). The interpretation of the results on each of the scales was done using established international methods.
Results
The present study was carried out at the gynecological endocrinology clinic of the Paulista Medical School from July 2018 to October 2019, when the target number of patients was reached. Initially 52 female patients were randomly divided into two groups: Group A (use of placebo) and Group B (use of transdermal testosterone nanoemulsion 300 mcg/day). Three patients were excluded for not meeting the inclusion and exclusion criteria: one with a BMI above 35 kg/m2, one with another cause of sexual dysfunction, and one for inconsistent data. This resulted in a total of 49 participants in the study.
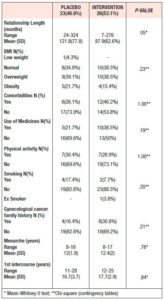
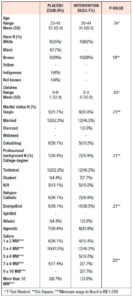
Baseline sociodemographic and clinical characteristics of the patients are presented in Tables 1 and 2. The groups had similar baseline characteristics.
Changes in FSFI Score
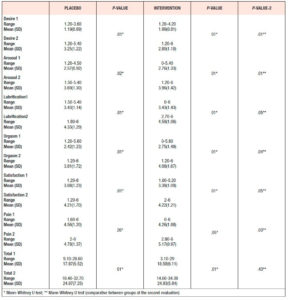
Both groups showed statistically significant increases in the total FSFI score after 12 weeks: the Placebo Group had a baseline mean score of 17.9 which rose to 24.1 after treatment, and the Intervention Group had a baseline mean score of 18.6, increasing to 24.9 after treatment. The Intervention Group showed a higher total FSFI score than the Placebo Group at the second timepoint (p> 0.05) (Table 3).
When the FSFI domains were evaluated independently, it was found that the Intervention Group obtained higher scores in most of all FSFI domains, when compared with the Placebo Group at the second timepoint, with statistical significance. (Table 3) Further, in all subdimensions of the FSFI except pain, it was observed in the Intervention Group that the youngest patients had the highest scores, which means the lowest risk of developing sexual disorder (p <0.05).
Changes in EATA score
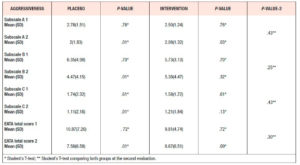
Evaluating the mean total EATA score at the second timepoint in each group, we observed a reduction in the values after 12 weeks. The Placebo Group had a mean score of 10.9, falling to 7.6 at the second evaluation (p <0.0.5) and the Intervention Group had a mean score of 9.8 passing to 8.7 at the second evaluation (p> 0.05). No significant differences were found between the groups in the total mean score or in the single subscale scores (Table 4).
Laboratory analysis
With regard to the analysis of serum total testosterone (Table 5) and free testosterone (Table 6), at the second evaluation of laboratory samples the mean values of the Intervention Group were found to have increased, however there was no statistical difference between the two timepoints or between the two groups after the treatment.
Analyzing the mean values of SHBG, we observed an increase at the second evaluation in the Placebo Group compared with a decrease at this timepoint in the Intervention Group. In neither group was the change statistically significant; furthermore, no significant difference emerged when comparing the two groups (Table 7).
Discussion
HSDD is one of the four most common forms of sexual dysfunction defined by the fifth edition of the DSM, and it is characterized by a chronic or recurrent deficit or lack of sexual desire, which causes distress and personal suffering35.
In this study, both groups showed increased total FSFI scores after the intervention; a placebo response is in fact known to be very common in trials that use subjective patient-reported measures like this one. Thus, a significant change above the placebo response can be taken to reflect a treatment effect, as seen in the group using testosterone, versus the Placebo Group, at the second timepoint in most of all FSFI domains, which showed statistical significance. Furthermore, this increase was even more evident in young patients.
Similar results were seen in a study by Shifren et al.23 where, despite an appreciable response to the placebo, the use of testosterone 300 mcg resulted in even greater increases in the frequency of sexual activity, sexual fantasies and masturbation, with increments of two to three times from baseline. Reduced suffering and distress due to greater sexual satisfaction were also observed, reaching 20% superiority when compared with placebo36.
A placebo response similar to or only slightly less than that observed with testosterone could also be associated with the circumstance that participants wished their sex lives to be more satisfactory; it may also be due to the fact that they felt welcomed by the researchers and the research team; along the same lines, participation in the trial may have encouraged greater communication with partners35.
In the present study, comparison of serum androgen levels between the groups at the second timepoint revealed higher values in the Intervention Group, but without the differences reaching statistical significance. Also, when we compared the Intervention Group before and after the use of testosterone, we observed increases in total testosterone and free testosterone and a decrease in SHBG, again without the differences reaching statistical significance. This same trend in hormonal profile was observed by other authors, within the same treatment period, in studies with larger numbers of participants, and similar or even higher testosterone concentrations17,18. This means that testosterone does not interfere negatively in laboratory tests, demonstrating safe use.
In addition, physiological free testosterone levels closer to the upper limit in the premenopause after the use of transdermal testosterone were found to be associated with an increase in the frequency of satisfactory sexual events when compared with placebo, again with good tolerability18.
Even so, neither our study nor others with a similar design, when compared placebo users with testosterone users, showed more cases of virilization, acne, allergic reactions, androgenic alopecia or voice alterations with statistical significance10,11,20,36 . No serious adverse effects occurred.
When evaluating the EATA scale results, the participants showed a reduction in the total score and in the subscale scores after the intervention, which indicates that use of testosterone 300 mcg was not associated with the development of behavioral changes characterized by a tendency to aggressiveness. Trials even show improvement in depressed mood and psychological well-being when using the same formulation compared to placebo10,11,17, probably because restoration of sexual health generates motivation and contentment, and vice versa20.
Thus, as demonstrated in our research, testosterone can be used in doses low enough to improve the patient's symptoms, while keeping serum androgen values as close to the physiological levels as possible, with minimal side effects.
Therefore, it is recommended to use the transdermal route with formulations in cream, gel, or transdermal patch according to availability, avoiding use for over 6 months in unresponsive patients, or in any case for over 24 months due to lack of available safety data.
Serum testosterone levels have not yet been clearly related to sexuality, behavioral changes or aggression. Serum testosterone levels should be monitored before the start of therapy, 3-6 weeks after starting the medication, and thereafter every 6 months, or also as necessary to make sure androgen levels are close to the premenopause normal physiological range10,11,36.
Despite several studies involving the use of testosterone to enhance desire and arousal, we have not yet been able to define the impact of this hormone on human sexuality in premenopause, as the trials differ in terms of the drug type, dosages and routes of administration, in addition to demonstrating benefits also with use of the placebo. Due to the small sample size and short patient follow-up period in this study, it is important to carry out complementary trials to gather more information on the safety and effectiveness of testosterone long-term therapy in the premenopause.
Conclusions
The transdermal Biolipid/B2® vehicle formulation with testosterone 300 mcg was shown in a 12-week treatment period to be safe and effective for HSDD patients in the premenopause, keeping plasma androgen levels within the normal range, demonstrating positive effects on sexuality, and having no negative effects on behavior, as shown by in the absence of tendency to aggressiveness.