Introduction
Endometriosis is one of the most common gynecological diseases in women of reproductive age. Over the last three decades, numerous basic and clinical studies have shown the complex pathogenesis of the disease, providing indications for clinical management (1,2). Several scientific societies have developed classification systems based on the appearance and extension of endometriosis at surgical exploration, and these have received general consensus worldwide. They have been developed on the basis of anatomical features, while some correlate with infertility, aiming to be quantitative and simple tools for doctors and patients (3).
Sampson classified ovarian endometriosis as a subgroup of hemorrhagic cysts of the ovary, proposing an etiology for the disease (4, 5).Observing the histologic appearance of endometrial-like glands and stroma in several ovarian hemorrhagic cysts, he added a fourth variety of ovarian hemorrhagic cysts to the previously known categories (follicular, corpus luteal, and stromal cysts).
In 1949, Wicks and Larsen proposed a classification for endometriosis based on histologic features (6), whereas Huffman's classification (7) was the first system based on surgical staging. Taking into consideration the localization and the extension of endometriotic lesions detected during surgery and pregnancy rates, he suggested adopting a more conservative approach in low grade stages. Later, another classification was proposed, based on the assumption that pelvic pain was caused by older deep fibrotic lesions. According to that, the disease develops in three stages: (a) early development, (b) active stage, and (c) relative endometrial inactivity (8).
In 1974, Mitchell and Farber developed a staging system similar to that used in gynecologic malignancies, including a stage V for malignant transformation to adenocarcinoma. The staging was also applied to determine whether to plan medical or surgical treatment (9).
In same years, a new classification system was proposed dividing endometriosis into three stages: mild, moderate and severe (10). It was based on the principle that the success of surgery in infertile women depended primarily on the severity of disease at the time of initial diagnosis.
In 1977, Kistner et al. (11) developed a classification system based on the natural history of the disease, from early peritoneal implants to ovarian involvement, tubal-ovarian disease and finally extension to the whole pelvis. Subsequently, Cohen proposed a ten-stage system based on the severity of laparoscopic findings. Extrapelvic endometriosis involvement, adenomyosis, and pelvic inflammatory disease were also evaluated (5,12).
Since none of the previous classifications had been universally accepted, in 1979 the American Fertility Society (AFS) generated an innovative new classification based on the use of a system of weighted values to report involvement of the peritoneum, fallopian tubes, and ovaries. The sum of the score gave the disease stage. To facilitate the description of the implants, a schematic representation of the pelvis was provided. This classification was revised in 1985 and 1996,giving rise to the Revised American Society for Reproductive Medicine score (rASRM) (13).
With respect to the rASRM, the ENZIAN staging system (2005) added information regarding the retroperitoneal structures and lesions localized in other organs (14, 15).
Later, in 2010, a further classification system called the Endometriosis Fertility Index (EFI) was developed, for predicting pregnancy rates in patients with surgically documented endometriosis who attempt non-IVF conception (16). In 2012, the American Association of Gynecologic Laparoscopists proposed a classification based on surgical difficulties, categorized into four levels (17).
Further endometriosis classification proposals were advanced by Batt et al. (18), Adamyan (19), Chapron (20), Martin (21), and Koninckx et al. (22). However, endometriosis staging remains an open field, especially since the last decade has brought new discoveries and insights that have changed the diagnosis and treatment of endometriosis.
The aim of the present review is to illustrate the history and the state of the art of the most widely used international endometriosis classifications, evaluating the strengths and limitations of each. In addition, considering the most recent research data, the newly proposed classification/staging systems are described and correlated with the most common clinical manifestations.
Methods
The PubMed, Medline, Embase, and Web of Science databases were searched to identify relevant studies on the classification of endometriosis from 1949 to 2019. The search terms included “endometriosis”, “staging” and “classification” in combination with “prognostic value”, “surgery”, “infertility”, “pain” and “diagnosis”. Only studies in English that were published as full-length articles were considered, excluding case reports. We analyzed the titles and abstracts of the 1471 results produced by the search, selecting only articles that referred to surgical classifications and imaging staging systems.
Classification systems
-
The Classification of the American Society for Reproductive Medicine (rASRM)
The rASRM classification system is based on intraoperative disease findings, and it takes into account peritoneal endometriosis, ovarian endometriosis, posterior cul de sac obliteration, ovarian adhesions and tubal adhesions (13). In particular, scores are assigned to endometriosis lesions in the peritoneum and ovaries using points that correspond to the size of the lesions. By analogy, points are also assigned for adhesions on the ovaries and Fallopian tubes. Additional points are assigned for partial or complete posterior cul-de-sac obliteration.
Finally, the assigned points are summed and a value is obtained, classifying the disease in one of four stages:
- Stage 1 (Minimal Endometriosis): 1-5 points;
- Stage 2 (Mild Endometriosis): 6-15 points;
- Stage 3 (Moderate Endometriosis): 16-40 points;
- Stage 4 (Severe Endometriosis): >40 points.
The rASRM endometriosis classification system is the most widely used worldwide. For health providers, it is very easy to apply, and for patients, it is easy to understand. However, it has some limitations. In fact, it is an arbitrary scoring system based on subjective score allocation and it has wide score ranges between the different categories. Furthermore, the stages do not provide any information about disease morphology. The rASRM has poor reproducibility if the disease involves the ovaries and the posterior cul-de-sac. Furthermore, given the various presentations of the disease, observer variability may be present, leading topossible problems in documentation. The scoring system can be affected by surgical technique (laparoscopy or laparotomy) and by the timing of surgery. In addition, it does not take in consideration the possible time-related evolution of lesions or hormonal treatments. Moreover, deep infiltrating endometriosis (DIE) and retroperitoneal structures are not adequately described (12, 23-25).
There is a very poor correlation between the extent of disease expressed by rASRM score and pain symptoms, infertility or patient quality of life. As regards prognosis, there is no correlation with infertility outcome and only poor predictive accuracy of treatment outcome.
Therefore, the rASRM endometriosis classification system gives poor prognostic information (12, 23-25).
-
The ENZIAN classification
The ENZIAN classification was developed as a supplement to the rASRM score, in order to provide a morphologically descriptive classification of DIE, taking into account retroperitoneal structures (15).
In this classification retroperitoneal structures are divided into three compartments:
- Compartment A: vagina, recto-vaginal septum;
- Compartment B: uterosacral ligaments to the pelvic wall (BB: bilateral involvement);
- Compartment C: rectum and sigmoid colon.
Disease severity is classified as:
- Grade 1: invasion <1 cm;
- Grade 2: invasion 1-3 cm;
- Grade 3: invasion >3 cm
Deep endometriosis invasion beyond the lesser pelvis and invasion of organs are recorded separately:
- FA: adenomyosis;
- FB: bladder invasion;
- FU: intrinsic ureteral endometriosis;
- FI: bowel disease cranial to the sigmoid colon;
- F0: other locations.
The prefix "F" stands for "far" or "foreign", referring to distant retroperitoneal structures.
The ENZIAN classification nomenclature, which is similar to the TNM (Tumor, Lymph Nodes, Metastasis) staging system used in oncologic diseases, is the following: A0–3 B0–3 C0–3 FA, FB, FU, FI, FO. Distant locations are only stated when present. When more than one focus is present in each compartment, only the largest is evaluated.
The ENZIAN score, describing DIE, can be considered complementary to the rASRM one. The advantages of the revised ENZIAN classification are related to the precise description of involvement of retroperitoneal structures,and the fact that DIE lesions can be described pre-operatively.
The revised ENZIAN classification system is mainly used in German-speaking countries, but has poor international acceptance. It does not take into consideration the morphological characterization of the lesions and it is more complicated, both for patients and for clinicians. With regard to prognosis, the revised ENZIAN score has poor prognostic value in terms of course of symptoms, quality of life, and response to infertility or pain treatment (15, 23-27).
-
The Endometriosis Fertility Index (EFI)
The EFI aims to predict pregnancy rates in patients with surgically documented endometriosis who attempt non-IVF conception. The EFI is a scoring system that includes assessment of historical factors at the time of surgery, of adnexal function at conclusion of surgery, and of the extension of endometriosis.
The following surgical findings are considered: the rASRM endometriosis lesions score (i.e., not including adhesions), the total rASRM score, and a functional score determined by the surgeon for each of the tube, fimbria and ovary bilaterally (normal, mild dysfunction, moderate dysfunction, severe dysfunction and absent or not functional).The historical factors considered are: patient age (≤35 years old, 36-39 years old, ≥40 years old), duration of infertility (≤3 years, >3 years) and prior pregnancy (history of prior pregnancy, or not) (28,29).
The surgical findings and historical factors each give a score. The two scores are summed to obtain the EFI score. The EFI score ranges from 0 to 10, with 0 representing the poorest prognosis and 10 the best prognosis. It is to be emphasized that the least function score is determined at completion of the surgical intervention, not before. It represents an estimate of reproductive functionality after the surgical intervention. The estimated cumulative percentage pregnant is presented graphically for each EFI score value. The EFI can be useful for predicting fertility outcome in women with previous surgical staging of endometriosis and can be useful in developing treatment plans for infertile women with endometriosis. Despite showing good correlation with spontaneous pregnancy rate, it does not consider uterine abnormalities, and does not correlate with pain symptoms (4,23, 25, 29).
Another consideration regarding the EFI score is that, by including infertility factors partially independent from endometriosis such as age, duration of infertility, and prior pregnancy, it obviously works in infertile women, but it is difficult to assess how much of its predictive value is related to the presence of different endometriosis forms.
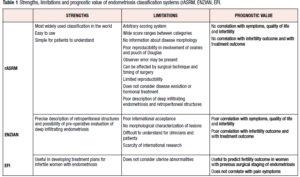
Table 1 shows strengths and limitations of each classification system, considering the prognostic value of each.
New perspectives: clinical staging of endometriosis based on imaging.
Other than those three recognized classifications there are several endometriosis staging proposals based on imaging. Transvaginal sonography (TVS) is considered the first imaging approach for diagnosis, staging and follow-up of endometriosis. The use of ultrasound imaging has several advantages: it is minimally invasive, cheap, readily available and acceptable to women; it provides a rapid result; it is a dynamic and interactive exam that makes it possible to evaluate the mobility of some structures and painful sites (30). Several studies have confirmed the high sensitivity and specificity of TVS in the diagnosis of endometrioma (31-34). Instead, a recent Cochrane review showed that the sensitivity and specificity of TVS are more heterogeneous in the diagnosis of DIE than in that of ovarian endometriosis: the lack of standardized definitions in the sonographic classification and diagnosis of DIE is a general cause for concern (35).
While the importance of ultrasound in the diagnosis of endometriosis is increasingly recognized, the challenge of developing a comprehensive and reproducible preoperative classification system for endometriosis nevertheless remains. The main problem in developing an ultrasound classification of endometriosis is the lack of a universal, systematic, evidence-based, and reproducible diagnostic protocol.
The International Deep Endometriosis Analysis (IDEA) group (36) published a consensus opinion shared by clinicians, gynecological sonologists, advanced laparoscopic surgeons and radiologists with an interest in endometriosis diagnosis and management. The group proposes four basic sonographic steps when examining women with suspected or known endometriosis, in order to systematically evaluate localization and extension of ectopic endometrial lesions:
- Routine evaluation of the uterus and the adnexa reporting the possible presence of adenomyosis and endometrioma
- Evaluation of transvaginal sonographic “soft markers” (site-specific tenderness and ovarian mobility)
- Assessment of the status of the pouch of Douglas (POD) using the real-time TVS-based ‘sliding sign’
- Assessment for DIE nodules in the anterior and posterior compartments.
A possible limitation is the operator's experience, especially in evaluating the sliding sign to predict POD obliteration and the severity of deep pelvic disease. Experienced operators who have performed more than 2500 scans reach proficiency in the detection of rectal DIE nodules and POD obliteration using TVS after approximately 40 examinations (37, 38).
Coccia et al. (39) proposed a staging system of DIE based on the evaluation of five components:
- Location (anterior, posterior, or lateral compartments);
- Size (longitudinal, anteroposterior and transversal axes of the implants);
- Shape: nodules (solid hypoechoic nodule with a rounded shape), linear thickening (abnormal hypoechoic linear thickening), or plaques (hypoechoic areas with irregular shape);
- Symptoms aroused during the exam:none (0), mild (1–3), moderate (4–6), and severe (7–10);
- Infiltration of the bowel wall.
The authors also evaluate the presence of monolateral or bilateral ovarian endometrioma, kissing ovaries, adenomyosis, and fixity of organs, as well as the urinary tract. The main limitation of this system is the difficulty in evaluating symptoms: in fact, the perception of pain might vary from individual to individual and in the same individual; the pain depends on the pressure exerted by the examiner with the probe and it is difficult to identify which lesion/s is/are responsible for pain in the case of multiple lesions.
The Endometriosis Surgical-Ultrasonographic System (ESUS) (40) is a preoperative mapping of endometriosis, developed to record the location, size, and depth of lesions visualized preoperatively by TVS and subsequently confirmed by laparoscopy and histology. The ESUS was compiled by marking the location of pelvic endometriosis divided into four compartments (adnexal, anterior, posterolateral, and Douglas) and by selecting, for each lesion, the corresponding box option of ‘‘yes-no’’,also adding the relative diameter and depth of infiltration. The authors reported variable diagnostic accuracy, ranging from 76 to 97% depending on the anatomical site: the lowest accuracy (59%) was obtained in the diagnosis of vaginal endometriosis, whereas the greatest accuracy (97%) was shown in detecting bladder lesions and Douglas obliteration. The ESUS systematic evaluation of the different pelvic sites is an easy process for both ultrasonographer and surgeon. The main limitation of this study was the high prevalence of DIE, representing a possible source of bias, due to the patient selection in three endometriosis referral centers.
Menakaya et al. (41) developed the ultrasound-based endometriosis staging system (UBESS), a score designed to predict the level of complexity of laparoscopic surgery for endometriosis, in order to facilitate referral of women with higher-stage endometriosis to tertiary laparoscopic centers. Used for TVS examination, this ultrasound-based approach consisted of(42):
- routine assessment of the uterus and ovaries,
- tenderness-guided assessment of the pelvis
- assessment of organ mobility including assessment of ovarian mobility (IIIa) and assessment of POD status (IIIb)
- assessment of anterior, lateral, and posterior pelvic compartments for non-bowel DIE
- assessment of the anterior wall of the bowel for bowel DIE.
Based on the ultrasound data from this five-domain model, the authors developed their three-stage preoperative UBESS using the Royal College of Obstetricians and Gynaecologists recommendations on the stratification of complexity of laparoscopic procedures (43). UBESS stage I (UBESS I) predicts mild disease and the need for a level 1 trained laparoscopic surgeon. UBESS stage II (UBESS II) predicts moderate endometriosis and the need for a level 2 trained laparoscopic surgeon, while UBESS stage III (UBESS III) predicts higher stage (severe) disease and the need for a level 3 trained laparoscopic surgeon. UBESS showed an accuracy of 84.9% in predicting the exact level of laparoscopic surgery and performed best in predicting severe endometriosis. The main limitations of UBESS is that it was developed and applied retrospectively and has been applied in women with a high prevalence of endometriosis referred to tertiary centers with high experience in endometriosis ultrasound diagnosis.
Magnetic resonance imaging (MRI) is a reliable preoperative diagnostic procedure that allows both localization of endometriosis lesions and planning of the surgical procedure, in particular for DIE. International consensus reports regarding preoperative MRI diagnostic protocols in DIE are sparse.
Zanardi et al. (44) proposed a staging of pelvic endometriosis based on MRI features, and compared it with the AFS laparoscopic classification. The MRI score was based on size, edges, wall thickness, septations, signal intensity on T2-weighted images of endometriomas, and presence of pelvic implants. This score classifies endometriosis in four classes, comparable with those of AFS laparoscopic staging. There was agreement between the MRI and AFS classification in 33/35 patients, and thus only two cases of discordance. Two other studies (45, 46) compared preoperative MRI features with intraoperative surgical results in patients with DIE using the ENZIAN score and found an excellent correlation with the intraoperative findings. However, standardization of MRI protocols used in the detection of DIE will be a crucial step towards increased diagnostic validity.
Recently, a preoperative score based on TVS and MRI showed good accuracy in predicting the risk of recto-sigmoid endometriosis (47).
The development of an imaging classification of endometriosis is a possible future perspective. However, a universally accepted diagnostic protocol would be necessary in order to map the disease, triage women to different forms of treatment, andfollow up the lesions. Furthermore, a shared protocol would help in evaluating the efficacy of a medical treatment, in identifying sites of the disease that could involve surgical risks and thus require a multidisciplinary approach, and in producing a standardized method and language for scientific groups.
Prognostic value of endometriosis classifications/staging systems for painful symptoms, infertility and surgical planning.
Painful symptoms
Endometriosis is typically characterized by several painful symptoms, in particular dysmenorrhea, cyclic and non-cyclic pelvic pain, deep dyspareunia, and cyclic intestinal and urinary symptoms (48).
In 1996, Vercellini et al. (49) correlated pain symptoms, measured by visual analog scale, to rARSM stage. The authors did not find any correlation with acyclic pelvic pain, deep dyspareunia and dysmenorrhea. Similar results were detected by the same group in 2006, with the exception of acyclic pelvic pain, which was significantly associated with severe stage of endometriosis (50). In 2013, to confirm whether the revised ENZIAN classification correlates with clinical symptoms, especially with pain, Haas et al. (51) performed a prospective study. They found that ENZIAN correlated partially with clinical symptoms, in particular lesions in compartment A with abdominal pain and lesions in compartment C with bowel symptoms.
Moreover, abdominal pain and dysmenorrhea seem to be correlated with the higher stages of the disease (26). Although the ENZIAN classification system might correlate with pain and dysmenorrhea, it does not consider the level of pain.
Infertility
Although the rAFS is the most widely used staging system for endometriosis, it does not provide a good characterization of disease severity and pregnancy outcome (52). The EFI ist he only classification system to predict pregnancy rate (PR) after surgery in endometriotic infertile patients. This index has been validated as clinically useful among patients with surgically confirmed endometriosis who wish to become pregnant and has been validated externally in populations of infertile patients with endometriosis after surgery.
The EFI score was derived from a cohort of 579 patients and then prospectively tested in 222 patients, confirming that it predicts PR after endometriosis surgical staging (29). Other studies designed to validate the EFI score have been published since the original article by Adamson.
Wei et al. (53) carried out an external retrospective validation in 350 patients. The authors found a significant association between a high EFI score and the probability of conceiving spontaneously within 3 years (71.8% for scores of between 8 and 10 versus44.4% for scores of between 5 and 7). However, the article was published in Chinese and the limited number of women with a score of between 0 and 4 limits the validity of the results.
Tomassetti et al. conducted a retrospective cohort study in which the EFI was related to pregnancy outcomes in 233 women attempting non-assisted reproductive technology (non-ART) conception immediately after surgery. A significant relationship was found between the EFI score and the time to spontaneous pregnancy. For each increase of 1 point in the EFI score, the relative risk of becoming pregnant increased by 31%. The average EFI score in their study was8, reflecting a population with a good prognosis. Therefore, these results do not allow any conclusions to be drawn about bad prognosis groups (28). The same authors, in another study, recently confirmed the high reproducibility of the EFI, supporting its use in daily clinical practice as the principal clinical tool for postoperative fertility counselling and management of women with endometriosis (54).
Boujenah et al. (55) also demonstrated external validation of the EFI, in 420 infertile and endometriotic patients after laparoscopic surgery. The authors found that patients with high EFI scores had significantly higher non-ART PRs compared with patients with low EFI scores after 12 months of follow-up. Moreover, non-ART PRs were significantly higher for patients with complete endometriotic lesion removal (ablation, resection, or excision and adhesiolysis) compared with patients with incomplete removal. These data underline the importance of surgical results. The strategy of removing as much endometriotic tissue as possible and then referring patients for ART if they failed to conceive spontaneously within 12 months after surgery led to an overall PR (surgery and ART treatment) of 78.8%. A 2015 Italian study also found a significant association between the probability of pregnancy and the EFI score in a series of 104 patients (56).
Li et al. (57) conducted a retrospective study enrolling 345 endometriosis-related infertile women after laparoscopic surgery. Significant differences in spontaneous PRs between different EFI scores were identified: the higher the EFI score, the better the chances of spontaneous pregnancy. In particular, in women with an EFI score of 4 or less, the spontaneous PR was very low. Therefore, to achieve a higher PR, the authors suggest that in vitro fertilization and embryo transfer should be recommended inpatients with an EFI score ≥5 at12 months from surgery.
Finally, Maheux-Lacroix et al. (58) performed a retrospective study of 235 women attempting pregnancy after resection of moderate-severe (Stage III–IV) endometriosis. They found that a higher EFI was associated with better fertility prognosis: for women with an EFI of 0–2 the estimated cumulative non-ART live birth rate at five years was 0% and steadily increased up to 91% with an EFI of 9–10, while the proportion of women who attempted ART and had a live birth steadily increased from 38 to 71% among the same EFI strata.
Use of the EFI score seems valuable, allowing non-ART procreation to be considered in cases with a high score, and, especially, allowing patients with the most unfavorable prognosis after surgery to be more quickly oriented towards ART. Data suggest that it is not the severity of endometriosis based on rASRM stage that is of primary importance in predicting pregnancy, but rather adnexal involvement, including ovarian disease and extensive endometriosis. The least function score (the sum of those scores determined intraoperatively after surgical intervention that describes the function of the tube, fimbria, and ovary on both sides) seems to be the main significant contributor to the prediction of spontaneous pregnancy among all the factors involved in the EFI score (28, 55, 57, 58). The EFI takes into account surgical findings both pre-surgery (ASRM scores, essentially amount of disease) and post-surgery (least function score, essentially functional capacity post-resection), and also historical factors including age, duration of infertility, and pregnancy history. However, the EFI has some limitations. Although age is included in the calculation of the EFI score, the ovarian reserve is not taken into account. In addition, the EFI score does not include severe uterine abnormality and adenomyosis. Finally, the EFI does not consider other possible mechanisms of infertility in cases of endometriosis (peritoneal, follicular, implantation disorders),beyond tubal-ovarian alterations.
Women in whom complete resection of endometriosis could not be achieved, with clinically-significant uterine pathology including leiomyomas, adenomyosis, intrauterine adhesions or congenital anomalies, or those having repeat surgery, have poorer prognosis. Therefore, these factors can be used to further guide management decisions, especially in the presence of an intermediate EFI, for individualization of care (58).
Surgical planning
Considering the high complexity of endometriosis surgery, careful preoperative planning of the treatment is essential. The ENZIAN classification provides good information about morphological characteristics of lesions, the side and localization of DIE lesions, and the involvement of retroperitoneal structures (11).
Haas et al. (27), in 2013, developed a model for preoperatively predicting surgical difficulty on the basis of on the ENZIAN system. Using multiple regression analysis, they developed a model for estimating the operation time in minutes, assuming complication-free procedures. The estimated operating time is calculated in minutes by adding the constant (intercept) and regression coefficients of the relevant ENZIAN classifications. This formula can be used for both single and combined lesions. Considering this model, a small lesion of the uterosacral ligaments (B1, BB1) does not significantly alter the operating time, whereas lesions with horizontal extension (B2, BB2, BB3) often require often ureterolysis and the procedure is longer, especially in the case of bilateral involvement. With regard to bowel endometriosis, small intestinal foci (C1) do not always require a complete bowel resection. In the case of C2 and C3 lesions the operating time is longer, while there is no major surgical time difference if the intestine is resected for a 1-3 cm endometriotic nodule (C2) or for a larger nodule (>3 cm).
Considering this model, the ENZIAN score is useful not only as a supplement to the rASRM score; indeed, it is also highly suitable for precise planning of surgical management and for informing DIE patients regarding the planned operating time.
TVS can be useful in pre-surgical evaluation in order to plan the intraoperative management of patients with endometriosis, giving a good prognosis of the surgical difficulty. In particular, with ESUS, Exacoustos et al. (40) created an accurate preoperative mapping of pelvic endometriosis lesions using TVS. The authors demonstrated that pre-surgical evaluation performed by an expert sonographer using ESUS shows elevated accuracy in DIE diagnosis and characterization. It is useful for evaluating the presence and localization of DIE, helping the surgeon in the planning of endometriosis surgery (surgical approach, involvement of other specialists, communication with the patient, management of disease). Similarly, UBESS staging is useful for predicting the level of complexity of laparoscopic surgery for endometriosis, in order to facilitate the referral of women with higher-stage endometriosis to tertiary laparoscopic centers (41).
Finally, the ENDORECT score (47) is a simple preoperative score based on MRI and TVS that predicts the risk of recto-sigmoid endometriosis.
The score is based on four simple preoperative YES/NO items: palpation of a posterior nodule on digital examination, a UBESS score of 3 on TVS, rectosigmoid infiltration on MRI, and the presence of blood in the stools during menstruation. The score results in three recto-sigmoid endometriosis risk groups (high, intermediate and low) with good accuracy.
Endometriosis surgery needs an adequate classification system for use in pre-operative planning of the treatment and in informing patients. Among the existing classifications, only the ENZIAN score can contribute to pre-surgical evaluation of the operating time, based on the dimension and localization of the lesions. TVS can add information about the presence and localization of DIE and, if combined with MRI, can predict the presence of recto-sigmoid endometriosis.
Conclusion
The present analysis confirms that we have a great collection of classification systems for endometriosis. The use of a toolbox for surgical classification of endometriosis that includes the rASRM, ENZIAN and EFI staging systems has been also proposed, giving a picture of the surgically treated patient and prognosis for desire of pregnancy (23).
From the overall evaluations it is clear that, with the exception of the EFI which correlates with fertility outcome, the main limitation of current classifications is their poor prognostic value. In fact, in the last two decades it has become clear that: a) endometriosis is a chronic inflammatory disease (59); b) menstrual-related pain is a critical symptom and is not correlated with surgical staging (60); c) endometriosis surgery entails multiple recurrences (61); d) the diagnosis of endometriosis by imaging (ultrasound and MRI) has greatly improved and may in part replace the surgical diagnosis (36); e) women with endometriosis have comorbidities which reduce their quality of life and hamper the management of patients (62); f) the advances in medical treatments and in ART are offering clinicians new tools (63); g) precision medicine is progressing and new scientific societies and networks are generating emerging knowledge (2).
None of the existing classification systems predicts the degree of pelvic pain, the disease recurrence, the rate of associated adenomyosis, the risk of comorbidities and quality of life. Nowadays, it is evident that surgical diagnosis and treatment are not mandatory, and there is indeed a poor correlation between symptoms and extent of disease found at surgery. There is a need to develop a clinical and not only anatomical classification, which takes into account symptoms and imaging features. Several sonographic protocols have been proposed for the assessment of the pelvis in women with suspected endometriosis, but no universally accepted sonographic staging system yet exists (25, 39, 40, 41).
A staging system for endometriosis (as for any other human disease) should not only predict the individual response to different treatments and help in formulating a prognosis, but should also aid in defining patient populations with similar characteristics, so that investigators might be able to reliably compare the results obtained in different referral centers. This seems to be a crucial way of improving care for women with endometriosis and of adding robustness to quantitative reviews of the available evidence. However, to become popular and be used worldwide, a classification system should be simple, rapid to use, and inexpensive; it should also demonstrate internal and external validity.
More importantly, any system must demonstrate internal and external validity in populations with pain, infertility, or both, and this is not the case with any of the currently available systems. Developing such a classification therefore seems to be a very, very difficult task, and only an international initiative might have some chance of succeeding.
It is not possible to design a reliable classification of a disease with unknown etiology and natural history, inconstant associations with infertility and pain, and variable response to medical and surgical treatment.
This review has evaluated the internationally accepted endometriosis classifications, focusing on the advantages and disadvantages of each. Its major strength is that it lists, in a simple manner, all the most used endometriosis classification systems, highlighting the prognostic value of each and identifying the situations in which they are applicable or not.
In conclusion, the existing classification systems of endometriosis were very useful in the past, but scientific and clinical information on the disease has now increased, modifying the management of these patients. Therefore, a new classification system with better prognostic values across all types of patients with endometriosis is warranted.