Introduction
Scar formation is the outcome of the tissue remodeling response to wounding. There are three distinct phases; an inflammatory phase which occurs within 48-72 hours after trauma and is driven by the release of cytokines and growth factors by damaged and dying keratinocytes and dermis cells, a proliferative phase which may last for up to 6 weeks, and a remodeling or maturation phase in which scars mature over a period of at least 1 year as the inflammation recedes and the collagen cross-links (1). Faulty healing resulting in excessive scarring is generally divided into two types: hypertrophic scars and keloid scars.
Hypertrophic scars remain confined to the borders of the original lesion. They follow the edges of the original wound but fail to appose its edges. They often are in areas of distracting tension. Hypertrophic scars may not recur when removed in a manner that releases skin tension (2, 3).
Keloid scars grow beyond the borders of the original wound, occur in non-tension areas and recur following removal (4). The mechanisms underlying keloid scar formation and their high recurrence rate after surgical removal are not well understood. One possible mechanism of pathogenesis is an alteration in extracellular matrix (ECM) metabolism resulting in an imbalance between its destruction and deposition, which may lead to excessive growth. Although the exact etiology is not well understood, this process likely involves a combination of both genetic and environmental factors (5, 6).
At present, the pathogenesis of both hypertrophic and keloid scars remains unclear. This has largely kept the treatment empirical (4).
Ezrin, radixin, and moesin (ERM) are membrane-cytoskeleton linkers that regulate a variety of cellular processes, including ECM interactions, apoptosis, cell-to-cell communication, carcinogenesis, and metastasis (7, 8). Among this group of proteins, ezrin is regarded as representative of the ERM family (9). Ezrin is expressed as a three-domain, folded protein whose active sites are not accessible until unfolding (activation) occurs. The intracellular pathway to unfolding ezrin most commonly is via the small Rho GTPase. The activated ezrin is welded (by phosphorylation) to the terminal intracellular domain of transmembrane receptors for a multitude of inflammation-related ligands including cytokines, growth factors and the hydrophilic glycosaminoglycan hyaluronic acid (hyaluran). The other domain of the activated ezrin may be similarly welded to structural proteins such as the actin cytoskeleton (9, 10). Thus, unfolded (activated) ezrin (p-ezrin) facilitates cytoskeleton-based cellular movement, formation of junctional complexes and regulation of organelle to organelle relationships (11). p-ezrin also can bind directly to intercellular adhesion molecules such as ICAM)-1 and ICAM-2, which co-localize with ezrin in microvilli projections and interact with the cytoskeletal protein β-actin (12). Recent studies have demonstrated overexpression of ezrin protein in many cancers, suggesting that ezrin may play an important role in tumor metastasis (10, 13-15). Expression of ezrin is induced by cytokines and estradiol (16-18), which may be relevant to the present study, see below. Despite these advances, the role of ezrin in the tissue remodeling attendant on wound healing remains poorly understood.
We investigated the role of ezrin in hypertrophic and keloid scars. Our study consisted of two parts; first, we used immunochemical methods to determine whether ezrin and p-ezrin participated in mouse wound healing. After these studies proved positive, we compared the expression of ezrin and p-ezrin in normal women’s skin, hypertrophic scar and keloid scar.
Materials and Methods
Expression of ezrin protein during wound healing of mouse skin
Performance of surgical wound and harvesting of healing skin edges in mice
All animal studies were approved by the Institutional Animal Care. Female 6-week old BALB/c mice (Oriental Bio) were anesthetized with 0.2 mL/kg zoletil 100 (Vribac Korea) combined with 9.8 mL/kg rompun (Bayer Korea). A 2.0 × 2.0-cm-sized skin lesion was made on the back of each mouse using surgical scissors. Epidermal, dermal, and subcutaneous tissues were removed, and the muscle fascia was exposed. To prevent wound contracture, eight 6-0 Vicryl sutures (Ethicon, Somerville, NJ, USA) anchored the wound margins to the underlying fascia. The animals were housed individually after surgery. Groups of 4 mice were euthanized after 7, 10, or 14 days, at which time full-thickness wound edge samples were removed, pegged flat and fixed in 10% neutral formalin, embedded in paraffin, and sectioned transversely at a thickness of 4 µm. Samples were then divided and examined histologically and following immunohistochemistry using antibodies against ezrin and p-ezrin.
Immunohistochemistry
Histology and immunohistochemistry
Histological analysis was performed after hematoxylin and eosin (H&E) staining. Immunostaining was performed using the EXPOSE Mouse and Rabbit Specific HRP/DAB Detection immunohistochemistry kit (ab80436, Abcam Inc., Cambridge, MA, USA) and antibodies specific for p-ezrin (1:200, Abcam, Inc., Cambridge, CA, USA) and ezrin (1:200; Abcam, Inc.). Samples were counterstained with Mayer’s hematoxylin. Negative controls were performed by omitting the primary antibody. For semi-quantitative analysis of immunoreactivity, an H-score was calculated by summing the percentages stained against the total area from five high power fields selected at random per section. Scores from two independent, blinded observers were utilized for each H-test.
Statistical analysis of H-testing - Data were analyzed using Fisher’s exact test and the Mann-Whitney U test, as appropriate. P values < 0.05 were considered statistically significant.
Human scar tissues
Sample collection
Study subjects were recruited from the Departments of Plastic Surgery and Obstetrics and Gynecology in a tertiary care university hospital. All samples were obtained after receiving written informed consent, and the study protocol was approved by the hospital’s institutional review board (SCHBC 2013-02-002). All subjects were women. Patient tissues were divided into three groups: normal skin (n = 8), hypertrophic scars (n = 7), and keloid scars (n = 7). Tissues were obtained by surgical excision at the time of unrelated surgery, cut into two parts with one half immediately stored at -80°C and the other pegged out and fixed in 4% paraformaldehyde.
Examination of tissues and H-testing were performed and evaluated as described above.
Protein extraction and sampling
Protein lysates were isolated at 4°C using pre-chilled buffers. The collected skin tissue was disrupted by mortar and pestle. After grinding the lysates were suspended in cold RIPA buffer containing freshly added protease- and phosphatase inhibitors and collected using a rubber policeman. The lysate was transferred into a 1.5 mL microcentrifuge tube and incubated on ice for 30 min, with gentle vortexing every 5 minutes. The mixture was then centrifuged at 13,000 × g for 20 minutes at 4°C to separate total proteins (supernatant) from the cellular debris (pellet). Supernatants were then transferred to a new tube for further analysis.
Western blotting
The supernatants’ protein concentrations were measured using a dye-binding protein assay based on the Lowry method. The protein lysate was separated by 10% SDS-PAGE after the lysates plus the house keeping protein β-actin were transferred electrophoretically onto a polyvinylidene fluoride membrane using standard techniques. The membranes had been blocked against non-specific binding by exposure to skimmed milk (5% [w/v] in TBS-T) for 1 h at room temperature. After washing three times with TBS-T, each membrane was incubated at 4℃ overnight with antibody to anti-p-ezrin or anti-ezrin plus anti-β-actin. Antibodies against p-ezrin and ezrin were purchased from Abcam; β-actin antibody was obtained from Sigma-Aldrich (A1978), see above. Secondary labelling antibodies were conjugated to horseradish peroxidase. Immunoreactive proteins were visualized using an enhanced chemiluminescence detection system on X-ray film.
Statistical analysis
Quantification of Western blot images was performed using the Image J program. Statistical analyses were performed using SPSS version 19.0 (SPSS Inc. Chicago, IL, USA). Data were analyzed using Fisher’s exact test and the Mann-Whitney U test, as appropriate. P values < 0.05 were considered statistically significant.
Results
Mouse
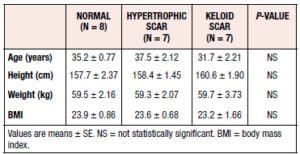
Ezrin and p-ezrin were present on immunohistological examination of repairing mouse skin. The wound edges exhibited time-related increases over time in expression of both intracellular ezrin and activated p-ezrin. Expression of these proteins was particularly increased around the regenerating epidermal layer and hair follicles.
H-testing - By day 10 post-operation, protein expression of ezrin, as calculated by the H-score, exhibited a 20.4% increase over day 7 levels. This trend continued to at least day 14, with ezrin protein expression 26.6% higher than that at day 7. The mean H-score of p-ezrin in post operation 14 day (POD14) was 28.80 ± 1.99 compared to post operation 10day (POD10, 22.87± 3.05) and post operation 7day (POD7, 11.08 ± 0.01). As well as, the mean H-score of ezrin in POD14 was 29.68 ± 2.46 more than POD10 (22.81 ± 0.53) and POD7 (11.16 ± 4.14). p-ezrin and ezrin levels were highest in the epidermis and hair follicles, Figure 1.
Humans
Clinical characteristics
All study participants were Asian women, with most tissues collected during term pregnancy cesarean section. Tissue samples were collected from 7 women with hypertrophic scars, 7 women with keloid scars, and 8 healthy controls. The most common site of tissue collection was the skin of the abdomen. The mean ages of the study participants in the control, hypertrophic scar and keloid scar groups were 35.2 ± 2.1 (S.E.) years, 37.5 ± 5.6 years, and 35.2 ± 2.1 years, respectively. The clinical characteristics of the study participants are listed in Table 1. No statistically significant differences in mean age, height, weight, or BMI were observed among the groups (Table 1).
Histological analysis of normal skin, hypertrophic scar, and keloid scar skin tissue
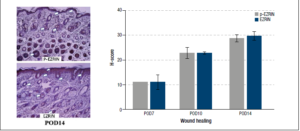
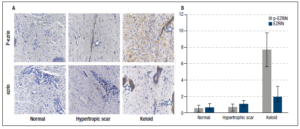
The formalin-fixed tissues were examined after H&E staining. Normal skin was composed of three layers; the epidermis and the dermis with the glassy subepidermal papillary layer giving way to a fibrous reticular layer and the hypodermis. Normal skin dermis and hypodermis were composed of multiple layers of fibrous tissue and collagen deposits. Hypertrophic scar tissue had similar epidermis and dermis to normal skin. Keloid scar epidermis was about as thick as normal scar but had few and thin papillae or rete pegs. Keloid had no papillary layer in the dermis, Figure 2.
Differences between ezrin expression and activation in normal skin, hypertrophic scar and keloid scars
Immunohistochemical analysis of ezrin and p-ezrin was performed for each group. Expression of both ezrin and p-ezrin was localized to the cytoplasm, with protein levels significantly higher in keloid and hypertrophic scar tissues. Because of the presence of separate tissue compartments in these layers, H-scores for the epithelium and sub-epithelium were obtained separately.
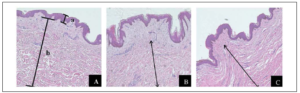
Ezrin in epithelium - The mean H-score of ezrin in normal skin was 1.70 ± 0.42, with much higher levels seen in both hypertrophic (7.32 ± 1.18) and keloid scars (8.08 ± 4.57). The differences in ezrin expression were significantly higher for both hypertrophic (p = 0.003) and keloid scar tissues (p = 0.037) compared with normal skin tissue. See illustration and H-test scores in Figure 3.
Activated p-ezrin in epithelium- The mean H-score of p-ezrin in normal skin tissue was 4.98 ± 1.12, compared with 5.90 ± 0.94 and 6.65 ± 3.12 in hypertrophic and keloid scar tissues, respectively; but, because of large variability in the tissue these differences did not reach statistical significance for either hypertrophic scar (p = 0.643) or keloid scar tissues (p = 0.728) relative to control normal skin tissue, Figure 3.
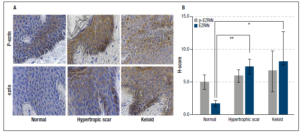
Ezrin in sub-epithelium - The mean H-score of ezrin in normal skin was 0.60 ± 0.26, with much higher levels seen in both hypertrophic (1.06 ± 0.20) and keloid scars (1.92 ± 0.64); however, because of high S.E.’s these scores were not significantly higher than normal skin, Figure 4.
Activated p-ezrin in sub-epithelium - The mean H-score of p-ezrin in normal skin tissue was 0.51 ± 0.19, compared with 0.63 ± 0.21 and 7.72 ± 1.01 in hypertrophic and keloid scar tissues, respectively. Despite variability in the tissues, the differences in p-ezrin expression were significantly higher for keloid scar tissues compared with normal skin tissue (p = 0.021). The differences were not significant for normal and hypertrophic scar (p = 0.386), Figure 4.
The presence of the extremely high level of activated ezrin in the keloid dermis fibroblasts and the infiltration of non-staining lymphocytes cells is seen in Figure 4.
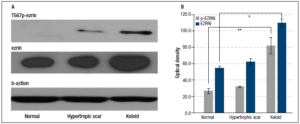
When the proteins from full thickness were subjected to western blotting, there were overall differences between normal skin and hypertrophic scar versus keloid tissue, the latter had significantly greater amounts of both ezrin and p-ezrin expressed. An example is shown in Figure 5.
Discussion
This is the first report of ezrin expression and activation in normal skin and abnormal scar tissue. The findings in mouse skin repair and over-expression in the hair follicle cells of mice and humans support the generalization of ezrin action across species and that ezrin may play a role in proliferation and replacement of the epithelial layer from outgrowing cells of the hair follicles.
Skin tissues undergo extensive remodeling and ECM deposition both immediately after surgery and during scarring. Ezrin has been shown to play an important regulatory role in cell polarity, shape, lateral specializations, motility and ECM rearrangement (7, 8, 19). Scar formation is mediated through such interactions, with hypertrophic scar and keloid formation representing imbalances in these responses to wounding (20). Since activated ezrin is an important regulator of cellular homeostasis, as well as cell invasion, migration, and cell junctional activities, our findings of the prominence and differential location of p-ezrin in the two types of abnormal scar are less surprising than indicative of the underlying pathology (7).
We showed that expression of both ezrin and p-ezrin proteins were increased in keloid scars, relative to normal skin, with expression localized primarily in the cytoplasm. While it is inappropriate to simply add them together to determine the overall expression of ezrin, it goes without saying that there could not be p-ezrin without ezrin expression.
Since there were significant differences in cellular and compartmental ezrin expression and activation between human normal skin, hypertrophic scar and keloids these findings suggest that ezrin may play different roles in the regulation of wound healing and scar formation and function. For example, compared to the findings in normal skin, the expression of ezrin and p-ezrin was dramatically increased in the lateral specializations of the hypertrophic scar epithelium.
The increase and distraction of the epithelial cell microvilli in hypertrophic scar may be an outcome of the tension on the epithelium. Surgical revisions rely on relieving these stretching influences for success (21), further indicating that tension along the re-epithelial line plays a role in the ezrin-directed construction of lateral specializations under these circumstances.
There is no papillary layer in keloids, just the loose connective tissue of the lower dermis. Ezrin and p-ezrin were most prominent in the fibroblasts of this layer. While involvement of ezrin in wound healing and tissue remodeling has been reported (7, 22), this relationship has not previously been explored in abnormal scar formation. But there are many proteins and hormones that regulate ezrin expression and activation and during scar formation these cellular products could play roles in development of the two abnormal phenotypes (2, 4, 6). For example, epidermal growth factor (EGF) and cytokines, including IL22, have been shown to play important roles in maintaining homeostasis during wound assembly (2, 3, 4, 6, 23, 24). EGF binding to fibroblasts regulates activation of ERM protein via the spingosine-1-phosphate pathway in cell invasion and migration (25, 26) and the p-ezrin could regulate cell proliferation, collagen assembly and many other aspects.
The greatest cellular concentration of ezrin protein in keloids was in activated ezrin (p-ezrin) in the dermis where an excess of inflammatory cells was found. This implicates the inflammatory cytokines and growth factors that have been mentioned in the literature as driving ezrin expression and activation (27, 28). EGF and IL22 are released by inflammatory cells and the dermis of keloids is infiltrated by lymphocytes. While the cause of this bland inflammation is not known, it seems a likely part of keloid growth and regrowth following surgical removal (29, 30). The large numbers of inflammatory cells in the dermis of keloids and the major activation of ezrin in this tissue supports the presence of continuing inflammation in the pathogenesis of keloids (15, 29).
Estrogen, tamoxifen and keloids - Recent reports of the effect of the estrogen antagonist selective estrogen receptor modulator (SERM) tamoxifen against keloids (31, 32) raise questions about the possible role of estrogen in keloids. We have shown that estradiol is a primary regulator of ezrin expression (18, 33). We also showed the presence of an aromatase-like molecule in lymphocytes (34), that was later confirmed to produce estrogen (35). Our results confirm the chronic state of inflammation in the dermis of keloids (5, 30). Estradiol and phytoestrogens have been shown to decrease EGF, IGF and cytokines and increase hydrophilic hyaluran expression and tissue hydration at the expense of hydrophobic GAGS, and to cause disruption of collagen rope integrity (36-39). Thus, it seems reasonable to posit that inflammatory cell-produced estrogen promotes the continued inflammatory state of the dermis of keloids and that tamoxifen’s effect is due to its estrogen receptor-blocking effect in keloid cells.
Limitations
(1) This is a preliminary, descriptive study. Therefore, the number of conclusions to be drawn are limited. For example, we cannot document the sources and actions of the differences in ezrin expression and activation inferred from these findings. Only further, mechanistic studies can do that.
(2) The expression of the two proteins is presented separately; the vagaries of immunohistochemistry and H-testing do not allow simple addition of results. Even the western blots of whole thickness biopsies are compromised in this regard because of the strong compartmentalization of the tissues. While generalizations regarding the functional implications of our findings may be made, combining the results to calculate the precise amount of ezrin protein in any specimen is not warranted.
(3) Enrollment in our study was limited to female subjects to minimize sexual dimorphic effects. The majority of the women were term pregnant, which may have influenced this study since pregnancy is a progesterone-dominated state that might have influenced expression of inflammatory molecules and estrogen receptor expression. (39, 40) While this may have affected the quantitative results, the positive descriptive morphological findings cannot be doubted.
(4) This study was limited to ezrin as a first approximation of ERM dynamics in scar. Other members of the ERM family have been shown to be active in tissue remodeling. A good example is Merlin/neurofibromatosis 2, abnormal forms of which play the main role in neurofibromatosis and several cancers. This ERM regulates contact inhibition, is a tumor suppressor and regulates growth factors. (41) Further studies of the possible roles of the complete ERM family in wound healing and scaring are warranted before the full picture in abnormal scaring is available.
Conclusions
ERM proteins have been an under-studied element in scar formation. The data presented here indicate that overexpression of ezrin and p-ezrin play important and biologically relevant roles in hypertrophic and keloid scars. The chronic bland inflammatory basis of abnormal scarring has been widely mentioned and is confirmed by our results. These studies further tie these forms of abnormal wound healing to estrogen and illuminate ways that tamoxifen may act against them.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3049819). This work was supported by the Soonchunhyang University Research Fund.
Competing interests
The authors declare no conflict of interest.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3049819). This work was supported by the Soonchunhyang University Research Fund.