INTRODUCTION
Menopause is associated with vasomotor symptoms such as hot flushes and night sweats, in addition to disorders such as sleep disturbances, anxiety, depression, decreased libido, sexual dysfunction, and poor memory. These conditions may require therapeutic intervention.1 Hot flushes are a disorder of thermoregulation caused by a lack of estrogens;2 this disorder involves brain neurotransmitters, which undergo important changes during the female menopausal transition.3 However, the underlying mechanism of hot flushes is not fully understood.
Increased levels of norepinephrine and serotonin have been shown to narrow the width of the thermoneutral zone (i.e., the space in which the thermoregulatory responses of sweating and shivering are balanced) and do not result in vasomotor symptoms.4,5 Small elevations in body temperature within an already narrowed thermoneutral zone lead to the vasomotor symptoms commonly associated with menopause.
Selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SNRIs) are an alternative to menopausal hormone therapy for the alleviation of vasomotor symptoms.6,7 In addition to the adverse effects generally associated with these molecules, they can potentially reduce the efficacy of tamoxifen treatment in women with breast cancer by markedly inhibiting CYP2D6, an enzyme necessary for to metabolize tamoxifen into its active form.8-10
Purified and specific cytoplasm pollen extract, PureCyTonin®, a nonhormonal alternative to menopausal hormone therapy, has demonstrated its efficacy and safety with respect to vasomotor symptoms in randomized, double-blind, and open-label clinical trials.11-15 This natural product has no demonstrable estrogenic activity16,17 and does not interfere with CYP2D68. Moreover, PureCyTonin® has also proven efficacious in women with premenstrual symptoms such as sleep and irritability, and it improves cognitive function 18-20.
The present study was designed to evaluate the potential of PureCyTonin® to inhibit serotonin reuptake in the rat brain model, and to discuss whether it might have a beneficial role as a non-hormonal option for the treatment of hot flushes, or other related brain-related symptoms of menopause.
METHODS
Purified and specific cytoplasmic pollen extract is a combination of purified pollen/pistil cytoplasmic extracts from selected plants belonging to the Gramineae and/or Pinaceae family. These plants are harvested separately using a standardized method. Purified and specific cytoplasmic pollen extracts are manufactured following a process that destroys the allergenic components of pollen. The allergens are broken down into peptides and amino acids, thus minimizing the risk of allergic reactions. Purified and specific cytoplasmic pollen extract was dissolved in water, and stock solutions were stored at 4°C.
5-[1,2-³H(N)]-hydroxytryptamine-creatinine sulfate NET-498 (serotonin), the SSRI fluvoxamine, and the irreversible selective monoamine oxidase (MAO)-B pargyline were purchased from Sigma. Sucrose (RdH 16104) was obtained from Sigma-Aldrich. Tris NaCl, KCl, D-(+)-glucose monohydrate, and ascorbic acid were purchased from Fluka. CaCl2*2 H2O and MgSO4*7 H2O were obtained from Merck. HEPES Sigma H 4034, Ultima Gold was obtained from Packard. Water was purified using a Millipore MilliQ purification system.
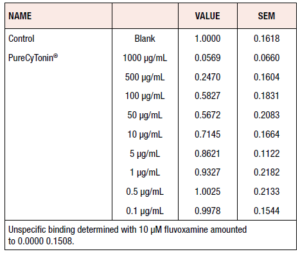
Synaptosomal preparations derived from whole brains of rats were used for serotonin reuptake as previously described.21-24 Cortex tissue was dissected fresh from male Wistar rats, immersed immediately in 10 volumes of ice-cold 0.32 M sucrose, and homogenized with a Potter-Elvehjem glass/teflon homogenizer. The resulting preparation was centrifuged at 900g for 10 minutes at 4°C. The pellet was discarded and the supernatant centrifuged again at 10,000g and 4°C. The supernatant was discarded and the pellet was stored with 0.32 M sucrose on ice until needed. Assays were carried out in physiological buffer (pH 7.4) (140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 11 mM glucose, 25 mM HEPES, 0.6 mM ascorbic acid) containing the MAO inhibitor pargyline at a concentration of 50 μM. Ten microliters of drug solution in DMSO (Dimethyl sulfoxide), nonspecific ligand in DMSO, or DMSO alone, 180 μL of buffer, and 50 μL of synaptosome preparation resuspended in buffer were added to each well of a 96-well filtration plate prewetted with buffer (Millipore Multiscreen). Each drug concentration was measured in 8 experiments. Incubation was for 15 minutes at room temperature, after which 10 μL of [³H]-serotonin solution in buffer (final concentration 2 nM) was added for a total volume of 250 μL. Incubation was continued for a further 20 minutes at 37°C, before termination by rapid filtration and washing with ice-cold buffer. Any radioactivity remaining on the filters was estimated with a liquid scintillation counter based on an efficiency of about 50%. Specific uptake is defined as total uptake minus binding in the presence of 10 μM fluvoxamine.
Statistical Analysis
Radioactivity accumulated in the filters was normalized to the mean of control experiments. EC50 values were determined by non-linear curve-fitting using the statistics software Sigmaplot® (SPSS Science Inc., USA). Values are given as mean value +/- CI95 from 8 experiments.
Cell lines
SHSY5Y cells (ATCC, Manassas, Virginia, USA) were maintained in a mixture 1:1 of Ham's F12 and Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/mL penicillin and 50 µg/mL streptomycin (0.5% P/S) in a humidified atmosphere of 5% CO2 in air at 37°C. The cell medium was replaced every 3 days. Cells were transferred to 6-well plates (seeded at 1e5 cells in 2 mL media per well) for RNAseq experiments and to 96-well plates (seeded at 1e4 cells per 0.2 mL media per well) for MTT assays. At ~50% confluence, the medium was changed to DMEM:F12 supplemented with 1% FBS, 0.5% P/S, and 10 µM all-trans-retinoic acid (ATRA) for 3 days to initiate differentiation. Cells were then treated for 24 hours with 400 µg/mL of PureCyTonin® in maintenance medium.
RNA isolation
RNA from the cells treated as described above (groups in quadruplicate: control and 400 µg/mL) was isolated using the Macherey-Nagel NucleoSpin RNA kit as per the manufacturer’s instructions and resuspended in RNase-free water.
mRNA-seq profiling
The purity and concentration of the RNA extracts were measured on an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, California, USA). A total of 200-500 ng of total RNA was used to prepare Illumina HiSeq libraries according to the manufacturer’s instructions for the TruSeq Stranded RNA kit (Illumina Inc, San Diego, California, USA). The mRNA template libraries were then sequenced as single pass 2´150-bp reads on the Illumina HiSeq4000 platform at the University of Colorado’s Genomics and Sequencing Core Facility. A custom pipeline was used for analysis of the sequence data, as follows: FastQC (v0.11.3) → Trimmomatic (v0.38) → FastQC → HiSat2 (v2.1.0) → SamTools (v1.8) → Stringtie (v1.3.4d) → DESeq2 (v1.20.0) → BiomaRt (v2.36.1). Reads were mapped to the H. sapiens GRCh38 genome_tran index provided by the developers of HiSat2. Fold-change and false-discovery rate were calculated using DESeq2, and gene names were annotated using BiomaRt.
RESULTS
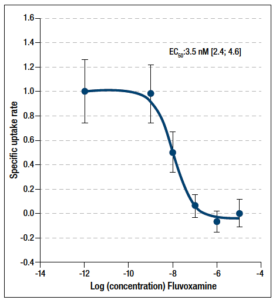
First, in order to assess the potency of the test system, the effect of the SSRI fluvoxamine was tested as a positive control. Fluvoxamine inhibits serotonin uptake in rat brain synaptosomes with an EC50 of 3.5 nM (Figure 1A).
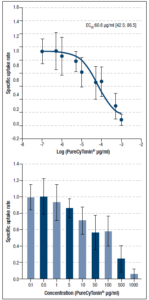
We then asked whether PureCyTonin® could influence uptake of [³H]-serotonin into rat cortical synaptosomes. Radioactivity accumulated in the filters was normalized to the mean of control experiments. The data obtained from the statistical analysis are expressed as fractions of control values (mean 95% CI from 8 experiments) (Table 1).
PureCyTonin® inhibited reuptake of [³H]-serotonin into rat cortical synaptosomes in a dose-dependent manner. The EC50 value was 60 μg/mL, which is well within a physiologically achievable order of magnitude (Figure 1B).
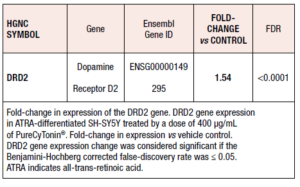
In order to further explore the effect of PureCyTonin® on the neuroendocrine system, we performed an RNA-sequencing study to obtain a better understanding of which pathways are modulated by pollen extracts and their correlation with therapeutic benefits. Neuroblastoma-derived SH-SY5Y cells differentiated by ATRA have long been used to study neuronal signaling pathways modulated by chemical entities. ATRA induces differentiation of these proliferating cells to cells that exhibit neuron-like properties, including expression of cholinergic neuronal markers, such as choline acetyl transferase, and dopaminergic markers, such as tyrosine hydroxylase and dopamine transporter. In ATRA-differentiated SH-SY5Y cells treated with purified and specific pollen extract, we found a significant increase in expression of the dopamine receptor D2 gene (DRD2) and other genes (Table 2).
DISCUSSION
During the menopausal transition, neuronal cells, neurotransmitters, neuropeptides, and neurosteroids in the central nervous system undergo important changes as a consequence of fluctuations in the levels of hormones such as estrogen.3 These changes could be responsible for the generation of hot flushes and modifications in behavior, cognition, and mood. Furthermore, evidence points to a neuroendocrine origin and suggests that a role is played by neurotransmitters such as glutamate, gamma-aminobutyric acid (GABA), serotonin (5-hydroxytryptamine [5-HT]), dopamine, acetylcholine, and noradrenaline, since some of the known treatments for these symptoms take the form of compounds that modulate the aforementioned neurotransmitters.6,7 Decreased estrogen levels have been shown to reduce serotonin levels and thus upregulate the serotonin receptor 5-HT2A in the hypothalamus. Additional serotonin is then released and can directly activate the 5-HT2A receptor. This activation could also change the temperature set point and result in hot flushes.25
SSRIs, which are administered for their effect on thermoregulation and sleep quality, act on serotonergic neurons. In general, both SSRIs and SNRIs cause adverse effects such as nausea, dizziness, headache, insomnia, sleepiness, and dry mouth. In breast cancer, these molecules have been shown to reduce the efficacy of tamoxifen by strongly inhibiting the enzyme CYP2D6, which metabolizes tamoxifen into its active metabolite.8-10
Premenstrual symptoms include psychological symptoms (e.g., anxiety, irritability, depression, loss of confidence, mood swings, and fatigue) and physical symptoms (e.g., bloating, water retention, and breast pain)Ces manifestations psychologiques et physiques peuvent rendre les femmes vulnérables et avoir un retentissement négatif sur leur vie professionnelle, sociale et sexuelle..26 Premenstrual symptoms are caused by changes in normal hormone levels, which can induce disorders in the central neuroendocrine system. D'ailleurs il est bien démontré que certains inhibiteurs de la réabsorption de la sérotonine ont une efficacité sur les troubles prémenstruels.SSSSerotonin can also play a role in these symptoms, as SSRIs have an impact on premenstrual disorders.27
In order to gain greater insight into the mechanism of action of PureCyTonin® with respect to menopausal symptoms and premenstrual symptoms, we analyzed the effect of purified and specific cytoplasmic pollen extract on the uptake of [³H]-serotonin into rat cortical synaptosomes. Purified and specific pollen extract inhibited the uptake of [³H]-serotonin into rat cortical synaptosomes in a dose-dependent manner. The EC50 value of 60 μg/mL is well within a physiologically achievable order of magnitude. Inhibition of serotonin reuptake may therefore play a role in the physiological action of PureCyTonin®. Additionally, the essential amino acid L-tryptophan is the precursor of the serotonin (5-hydroxytryptamine, 5-HT) present in the extract.
Interestingly, PureCyTonin® increases dopamine receptor D2 gene (DRD2) expression in ATRA-differentiated SH-SY5Y cells. It has been shown that estradiol activates the transcription of DRD2 in the frontal cortex and increases the level of DRD2 mRNAs. There is a substantial decline in DRD2 during an individual’s lifetime, and animal studies have shown age-related reductions in dopamine release and dopamine markers in the frontal cortex.28 Decreased levels of estradiol in postmenopausal women may lead to reduced expression of DRD2, thus contributing to the reduction of dopaminergic neurotransmission and impairment of cognitive and motor functions. Therefore, this increased expression of DRD2 induced by pollen extract may be beneficial for menopausal women.
One of the limitations of the study is that it was performed in an in vitro model using rat cortical synaptosomes. Although in vitro assays are attractive for elucidating the mechanism of action, one of their main disadvantages is their simplification of the in vivo situation: in vitro assays are mechanism-specific, whereas in vivo assays enable the detection of effects resulting from multiple mechanisms. Further studies should be done to measure serotonin content in healthy and menopausal donors. Taken together, our data show that PureCyTonin® is an effective inhibitor of serotonin reuptake in vitro and that it could have an effect on the DRD2 receptor pathway, which might in turn contribute to the beneficial effects observed in vivo. Further studies are needed to identify other molecular targets involved in the mechanism of action of PureCyTonin® (e.g., µ opiates, serotonin, and dopamine receptors).
CONCLUSION
Purified and specific pollen extract could act as an SSRI-like therapy without the adverse effects of currently prescribed SSRIs. Data obtained with our rat brain model indicate that pollen extract appears to maintain the availability of serotonin in hypothalamic serotonergic neurons, thus explaining, in part, its efficacy in controlling thermoregulation, sleep, and mood in menopausal woman. The modulation of other neurotransmitter pathways (e.g., DRD2) could also explain the mechanism of action of purified and specific pollen extracts such as PureCyTonin®.
Acknowledgments
Parts of this study were performed as subcontracted work at IBAM GbR, Denzlingen, Germany (Dr. Rainer Knörle and Dr. Peter Schnierle).
Conflicts of Interest
Santiago Palacios has been an advisory board member and symposium speaker on menopausal hormone therapy and tibolone, raloxifene, and bisphosphonates for Bayer Schering, Daiichi Sankyo, Eli Lilly, Exeltis, Gedeon Richter, MSD, Novo Nordisk, Procter & Gamble, Sanofi, Sérélys Pharma, Servier, and Teva. He has also received research grants and/or consulting fees from Amgen, Arkochim, Bayer Schering, Daiichi Sankyo, Eli Lilly, Exeltis, Gedeon Richter, Pfizer, Sérélys Pharma, and Servier. Kurt Appel works for VivaCell Biotechnology GmbH, a contract research organization.
Financial support for the analysis was provided by Natumin Pharma AB, the manufacturer of PureCyTonin®. The founding sponsors had no role in the design of the study or in the collection, analysis, and interpretation of data. The authors declare that they have no other conflicts of interest with respect to the present manuscript.