Introduction
Functional hypothalamic amenorrhea (FHA) is one of the main causes of secondary amenorrhea in women. FHA is the result of aberration of the pulsatile release of gonadotropin-releasing hormone (GnRH) associated with psychological, metabolic or physical stress, such as weight loss or excessive exercise1. FHA accounts for approximately 30-35% of secondary amenorrhea [amenorrhea for at least 6 months with very low serum luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels] and about 3% of primary amenorrhoea2. Impairment of GnRH pulsatile secretion in FHA leads to disturbances in the hypothalamic-pituitary-ovarian axis, which results in reversible ovarian dysfunction and profound hypoestrogenemia. Subsequently, hypoestrogenemia contributes to variety of dysfunctions occurring in FHA, such as cardiovascular disturbances, mental and sexual dysfunction, and reproduction failure. Amenorrhea and coexisting hypoestrogenism, leading to decreased peak bone mass, are risk factors for osteopenia and osteoporosis3. Women with FHA are also more prone to endothelial dysfunction, atherosclerosis and premature development and progression of coronary artery disease4. Disturbances of the normal cyclical changes in hormonal status are responsible for anovulation and impaired fertility. Moreover, FHA in the maternal anamnesis contributes to obstetric complications, such as miscarriage and preterm labor5.
It is reported that impaired secretion of FSH and, in particular, LH contributes to diminished GnRH pulsatile secretion. LH secretion disturbances in FHA range from total absence of LH secretion and a lower mean frequency or amplitude of LH pulses to a higher mean frequency of LH pulses.6 It is established that neurotransmitters and neuropeptides secreted in the central nervous system, such as kisspeptin, neuropeptide Y, corticotropin-releasing hormone (CRH), leptin, ghrelin, allopregnanolone and β-endorphin, play a particular role in the control of proper GnRH secretion7. Abnormal secretion of those substances is linked to the pathophysiology of FHA. Kisspeptin, a product of the KiSS-1 gene, plays a major role in pubertal maturation, acting on its specific G protein-coupled receptor GPR54. It is reported that mutations leading to impaired function of either kisspeptin or GPR54 lead to developmental abnormalities of the reproductive system and failure of pubertal progression8,9. On the other hand, elevated concentrations of kisspeptin or activating mutations of GPR54 result in premature thelarche and central precocious puberty, but not in premature adrenarche10-13.
Kisspeptin neurons are localized mainly in the preoptic area and arcuate nucleus (ARC), in humans also called the infundibular nucleus14,15. According to Hrabovszky,15 it seems that kisspeptin influences GnRH neurons in the median eminence of the hypothalamus and thus transmits signals from the ARC to the hypothalamic-pituitary-ovarian axis. In another region of the hypothalamus, the anteroventral periventricular nucleus, there is a positive correlation between KiSS-1 mRNA expression and estradiol oversecretion in the pre-ovulatory phase16.
The role of CRH in the pathomechanism of FHA is well documented17. Its action consists of direct and indirect (through β-endorphins) inhibition of GnRH. CRH may also inhibit kisspeptin secretion, which seems to underline its important role in the FHA pathogenesis. Therefore, it is CRH that has become an important element of this study.
The aim of this study was to evaluate hypothalamic-pituitary-ovarian dysfunction through analysis of hypothalamic hormones — serum concentrations of kisspeptin, CRH, LH, FSH, prolactin, estradiol and testosterone — in women with FHA and to compare the results with those recorded in a control group.
Materials and methods
Forty-one women aged 17-28 years were hospitalized at the Department of Gynecological Endocrinology at Poznan University of Medical Sciences. All the patients were thoroughly informed about the research and informed consent for their participation was collected. Each of the patients was diagnosed with FHA due to weight loss without coexistence of eating disorders. The patients fulfilled the following criteria:
- diagnosis of secondary amenorrhea, defined as amenorrhea for more than 90 days, unrelated to pregnancy,
- reduced serum LH (<5 mIU/ml).
- Exclusion criteria for the study group were:
- cranio-cerebral traumas, Sheehan's syndrome, or other syndrome with hypopituitarism, as disclosed in an interview;
- menstrual disorders with an etiology other than hypothalamic (hyperprolactinemia, hypopituitarism / hyperthyroidism, polycystic ovary syndrome);
- identification, during gynecological or ultrasound examination of the reproductive system, of morphological abnormalities within the reproductive system that may be the cause of primary or secondary amenorrhea.
The control group consisted of 40 healthy age-matched women who were screened according to the following criteria:
- regular menstrual cycles (menstruation every 28 ± 4 days) during the last 12 months,
- a normal body mass index (BMI) of between 18.5 and 24.9 kg / m2 and no history of weight loss in the past 12 months,
- lack of thyroid and adrenal function disorders,
- no hormonal treatment in the past 6 months.
Each patient was informed about the nature of the study and gave her written informed consent to participate in it. The consent of the Bioethics Committee of Poznan University of Medical Sciences for carrying out this study was also obtained (Resolution No. 982/07).
Anamnestic information was collected from the patients: age at menarche, any previous hospitalizations, treatments, occurrence in the past of severe stress or significant physical exertion, occurrence of weight loss in kilograms, obstetric history, chronic illness, medications, hormone therapy, past operations, drug sensitization, and family history.
In addition, all the patients underwent a physical examination, including:
- measurement of body weight, height;
- calculation of BMI [kg / m2], classified as follows: <18.5 kg / m2: undernutrition; 18.5-24.9 kg / m2: normal; 25-29.9 kg / m2: overweight; 30-39.9 kg / m2: obesity; > 40 kg / m2: huge obesity.
- gynecological examination,
- ovarian ultrasound examination (Aloka Prosound Alpha 6 vaginal probe, Hitachi Aloka Medical Ltd., Tokyo, Japan).
- The blood for hormonal examinations was collected in the fasting state. In the control group, blood was drawn in the follicular phase, at around days 11-13 of the cycle. The patient's blood serum was assayed by an enzyme-linked immunosorbent assay (ELISA). Serum kisspeptin, CRH, FSH, LH, prolactin, testosterone and estradiol levels were determined. Laboratory tests were performed at the Immunodiagnostic Laboratory and Radioimmunological Central Laboratory, Gynecological and Obstetric Hospital of the Poznan University of Medical Sciences, Poznan, Poland.
Statistical Analyses
The measurable parameters, such as kisspeptin, CRH, FSH, LH, estradiol, prolactin and testosterone concentrations, were reported as arithmetic mean and standard deviation. For the comparison between the two groups, Student's t-test was used for independent tests when data were consistent with the normal distribution, otherwise the Mann-Whitney nonparametric test was implemented.
Calculations were performed using the STATISTICA statistical package (v. 8.0).
Statistical analysis was performed at the Department of Computer Science and Statistics of Poznan University of Medical Sciences, Poznan, Poland.
Results
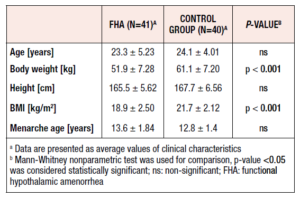
The mean body weight of the FHA patients was 51.9 ± 7.28 kg, which was statistically lower than that of the control group (61.1 ± 7.20 kg) (p < 0.001). These patients had a mean BMI of 18.9 ± 2.50 (kg/m2), which was statistically significantly lower than the mean value in the control group (p < 0.001). The mean BMI in healthy women was 21.7 ± 2.12 (kg/m2) (Table 1). The mean age at first menstrual period in the patients with FHA was 13.6 ± 1.84 years, whereas in the control group women it was 12.3 ± 1.45 years (p=0.281).
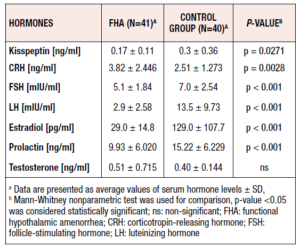
The mean serum concentration of kisspeptin in the patients with FHA was 0.17 ± 0.11 ng/mL, whereas in the control women it was 0.3 ± 0.36 ng/mL. This difference was statistically significant (p = 0.0271) (Table 2). The mean serum CRH concentration in the control group was 2.51 ± 1.273 ng / ml, versus 3.82 ± 2.446 ng / ml in the patients with FHA, in whom it was significantly higher (p = 0.0028) (Table 2).
Another parameter evaluated was the average serum FSH level in the patients versus the controls. The mean serum FSH level in the FHA patients was 5.1 ± 1.84 mIU/mL versus 7.0 ± 2.54 mIU/mL in the controls. The mean serum FSH concentration in the FHA patients was statistically significantly lower than the concentration recorded in the control group (p < 0.001).
Similarly, significantly lower values of serum LH were observed in FHA patients compared with controls (p < 0.001). The mean serum LH level in the FHA patients was 2.9 ± 2.58 mIU/ml, whereas in the control group it was 13.5 ± 9.73 mIU/ml. In addition, the FHA patients had significantly lower serum estradiol values compared with the healthy women (p < 0.001). The mean serum estradiol level in the FHA patients was 29.0 ± 14.8 pg/ml, whereas in the control group it was 129.0 ± 107.7 pg/ml.
Statistically significant differences in serum prolactin concentrations were recorded between FHA patients and control subjects. The mean serum prolactin concentration in FHA patients was 9.93 ± 6.020 ng/mL and was statistically significantly lower than that recorded in controls (15.22 ± 6.229 ng/ml) (p < 0.001). Finally, as regards mean serum testosterone levels, no statistically significant difference was found between the groups (p = 1.0). FHA patients had a mean testosterone value of 0.51 ± 0.715 ng/ml, whereas in the control group the concentration was 0.40 ± 0.144 ng/ml.
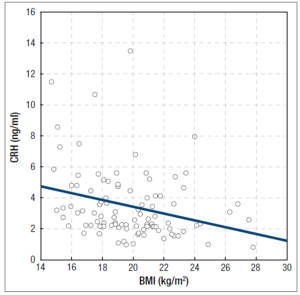
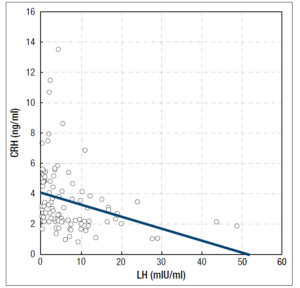
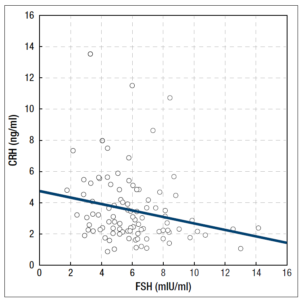
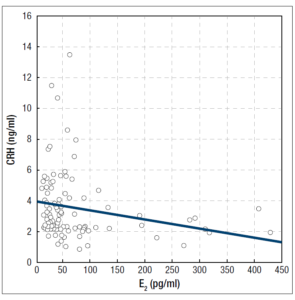
In the group of patients with FHA, we found several negative correlations: between serum CRH concentrations and BMI values (R = -0,305754, p = 0.002) (Figure 1), between serum CRH and LH concentrations (R = -0,438267, p < 0.001) (Figure 2), between serum CRH and FSH concentrations (R = -0,262760, p = 0.009) (Figure 3), and between serum CRH and E2 concentrations (R = -0,327471; p = 0.001) (Figure 4).
Discussion
The serum concentration of kisspeptin has previously been evaluated in physiological as well as in pathological conditions, and studies concerning serum kisspeptin concentrations in humans and animals can be found in the literature. In the present study, the mean serum concentration of kisspeptin in patients with FHA was assessed. Our data showed significantly decreased serum kisspeptin concentration in women with a diagnosis of FHA in comparison with healthy age-matched women. Nevertheless, previous studies suggest, that kisspeptin is secreted from the hypothalamus in a pulsatile manner, which, coupled with the short half-life time of kisspeptin and the fact, that kisspeptin is secreted also by peripherally located organs such as the pancreas, liver, small intestine, ovaries, adipose tissue and adrenal glands, may affect the conclusions drawn from the current trial. Nevertheless, previous studies suggest, that kisspeptin is secreted from the hypothalamus in a pulsatile manner, which, coupled with the short half-life time of kisspeptin and the fact, that kisspeptin is secreted also by peripherally located organs such as the pancreas, liver, small intestine, ovaries, adipose tissue and adrenal glands, may affect the conclusions drawn from the current trial18.
The results of the present study are consistent with previously performed investigations of kisspeptin concentrations in amenorrheic patients. In a previous study, Bacopoulou et al.19 revealed lower serum kisspeptin concentrations in amenorrheic adolescents with typical anorexia nervosa (AN) in comparison with healthy normally menstruating adolescents, although the difference was not statistically significant. Their findings are particularly significant given that AN is one of the factors that contribute the most to FHA development. Higher concentrations of kisspeptin were observed in patients with atypical AN when compared with healthy controls and with fully developed amenorrhea in typical AN19. In another study, similar results were noticed when menopausal status was taken into consideration. Plasma kisspeptin concentration was higher in perimenopausal women than in post-menopausal women and pre-menopausal women. Increased kisspeptin concentrations in AN patients and in perimenopausal women may be the result of excessive hypothalamic stimulation due to ovarian insufficiency. Nevertheless, in those states the ovarian impairment intensifies, which ultimately results in exhaustion of the kisspeptin neurons and a decreased plasma concentration20. The above evidence has accumulated in parallel with a recently conducted study on lactational amenorrhea. Kotani et al. revealed that plasma kisspeptin concentration is not significantly different in women with lactational amenorrhea when compared with healthy controls. Notably, in this study, plasma kisspeptin concentration in cases of pathological amenorrhea with very low FSH, LH and estradiol plasma levels was not completely reduced, which is an additional premise behind the above-mentioned hypothesis 21.
Jayasena et al.22 showed increasing LH pulsatility in women with hypothalamic amenorrhea using intravenous infusion of kisspeptin-54. Five patients were enrolled in the study. The researchers revealed that kisspeptin increased LH pulsatility in all patients with FHA. This work provided a basis for investigating a potential role of kisspeptin-related therapies in the treatment of women with FHA22.
In the present study, no correlation was found between serum kisspeptin and FSH, LH or estradiol concentrations. These observations are consistent with the results of studies reported previously, where no such correlations were found in healthy women or in AN 20,21. Other researchers found no correlation between serum kisspeptin and serum FSH and LH concentrations in postmenopausal women, premature ovarian insufficiency, and idiopathic hypogonadotropic hypogonadism23.
Since FHA in the enrolled group was caused mostly by dietary restriction, the study group had a significantly reduced BMI24. Kumar et al. revealed that dietary restriction in rats is associated with decreased concentration of kisspeptin mRNA expression and a significant decrease in hypothalamic kisspeptin25.
It remains to be clarified whether decreased kisspeptin concentration is a reason for impaired ovarian function, or whether insufficient production of ovarian steroids leads to decreased kisspeptin concentration. KNDy neurons located in the ARC and anteroventral periventricular nucleus (AVPV) express estradiol receptor α (ERα). Estradiol acting on ERα in KNDy neurons in the ARC suppresses GnRH and accordingly LH secretion. An adverse effect of estradiol is observed in the AVPV, where positive feedback probably responsible for preovulatory surge of LH occurs26. Moreover, kisspeptin neurons located in the ARC also express progesterone receptors, which suggests that both estradiol and progesterone play a particular role in exerting feedback on KNDy neurons26. It is believed that impaired secretion of kisspeptin, and subsequently GnRH, is the etiopathogenetic basis of FHA. Nevertheless, taking into consideration the previously mentioned data, it can be suspected that impaired secretion of ovarian steroids exacerbates abnormal KNDy neuron function. This issue requires further studies in the future.
With regard to CRH, there are relatively few papers in the literature describing the basic serum concentration of CRH in women with FHA. Extreme physical, nutritional, or emotional stress activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to increased secretion of hypothalamic CRH, corticotropin (ACTH) and adrenal cortisol. Stressors negatively affect reproduction throughout the hypothalamic-pituitary-ovarian axis. CRH inhibits GnRH pulse frequency and cortisol suppresses reproductive function in the hypothalamus and pituitary gland27. Women with FHA generally have higher basal cortisol levels and a blunted ACTH and cortisol response to CRH stimulation, likely secondary to negative feedback from hypercortisolemia and elevated ACTH. One study found that these blunted responses to CRH improved after menstrual recovery in FHA patients27.
Hohtari et al.28 published the first study assessing plasma CRH concentration in female athletes diagnosed with FHA. They collected 12 athletes with amenorrhea and 8 with a normal menstrual cycle. In addition to CRH concentration, the concentrations of ACTH and endorphins in the athletes’ blood plasma were also tested during rest and while cycling, requiring 80% and 100% maximal oxygen uptake. CRH concentration in blood plasma in sportswomen with amenorrhea did not differ during physical exertion from the values recorded in the group of sportswomen with a normal menstrual cycle. In both groups, there was a significant increase in both ACTH and also endorphins in the blood plasma in response to physical effort. In addition, higher concentrations of plasma endorphins during rest were found in sportswomen without menstruation compared with sportswomen with a normal menstrual cycle. Both the menstruating and the amenorrheic women were found to show the same response to physical effort in terms of concentrations of ACTH and endorphins.
In 2000, Meczekalski et al.29 studied the role of CRH in the pathophysiology of FHA. A role for the hypothalamic-pituitary-adrenal axis was suggested in FHA. 8 patients suffering from hypothalamic stress-related amenorrhea with normal body weight and 8 age-matched healthy controls in the follicular phase of the menstrual cycle were included in the study.
The blunted ACTH, allopregnanolone and cortisol responses to CRH suggested a reduced sensitivity and expression of CRH receptors in women with FHA29. According to a study by Takumi et al., stress-induced CRH inhibits GnRH and gonadotropin secretion via the kisspeptin system30.
In our study we reported increased serum concentrations of CRH in patients with FHA in comparison with healthy women. Moreover, we found negative correlations between CRH and BMI, FSH, LH and estradiol in patients with a diagnosis of FHA. All the results of our study confirm a non-physiological disorder of the HPA axis in patients with FHA.
In conclusion, our study revealed decreased serum kisspeptin and increased CRH concentrations in patients diagnosed with FHA in comparison with healthy age-matched women. This study should be followed by other studies on kisspeptin and CRH and their secretion patterns in FHA.
Acknowledgments
This study was supported by Grant of the Medical Faculty I, Poznan University of Medical Sciences, number: 502-01-01-11091360
Conflict of Interest Statement
The authors declare that there is no conflict of interest