INTRODUCTION
Although they are very effective in cancer management, chemotherapy and radiotherapy are highly gonadotoxic treatments that often result in premature ovarian insufficiency [1, 2]. Ovarian tissue cryopreservation and transplantation constitute the only currently available means of safeguarding fertility in young women who need to undergo treatment without delay, and also prepubertal patients yet to reach menarche [2]. Nevertheless, there are still various issues related to these procedures that need to be addressed, as massive loss of primordial follicles (making up the ovarian reserve) is known to occur after transplantation. This loss of dormant follicles is a consequence not only of follicle death due to immediate post-grafting hypoxia, but also of increased numbers of growing follicles [3, 4], suggesting that there is a dual mechanism at play: follicle death and follicle activation [5].
Quiescent primordial follicles reside in large numbers in the ovary. While most primordial follicles remain in a state of arrest due to dormancy factors, some start growing and developing to the primary stage under the control of serine/threonine kinases via two signaling pathways: protein kinase B (Akt) and mammalian target of rapamycin (mTOR) [6]. The phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/phosphoinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling cascade is indeed fundamental to a wide range of cellular processes, including nutrient metabolism, cell survival, cell growth and apoptosis [7]. Akt activation is regulated by the balance between PTEN and PI3K, which ultimately leads to varying degrees of Akt phosphorylation, hence triggering a number of pathways, including forkhead box O (FOXO) and the mechanistic target of rapamycin complex 1 (mTORC1) [8].
In ovarian tissue, the PI3K-Akt pathway, a signal transduction pathway, promotes survival, development and proliferation in response to oocyte- and granulosa cell (GC)-derived factors [6, 9]. Once activated, primordial follicles initiate growth and advance to primary and secondary stages under the influence of paracrine factors and, in time, follicle-stimulating hormone (FSH).
The Hippo pathway also appears to be implicated in follicle activation and growth through the action of Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) downstream effectors [6]. However, there is no conclusive proof that these pathways play a role in activating human primordial follicles, despite animal studies indicating clear involvement of these factors in primordial follicle activation in rodents [5].
Development of preantral follicles is curbed by the inhibitory Hippo signaling pathway [6, 10], so disruption of Hippo signaling promotes secretion of downstream factors capable of stimulating follicle growth [6, 9]. Disruption of this pathway (which occurs with ovarian tissue fragmentation), combined with stimulation of Akt signaling by phosphatase and tensin homolog (PTEN) inhibitors and/or PI3K stimulators, was indeed found to promote growth of preantral follicles [9, 11-13]. This combined method, known as in vitro activation (IVA), was proposed by Kawamura and Suzuki to stimulate the development of antral follicles in women with a diminished ovarian reserve [9, 13].
The objective of this review is to evaluate the true impact of disrupting the Hippo signaling pathway, associated with activation of the Akt pathway, on human ovarian tissue. What we need to establish is whether the Akt and Hippo signaling pathways play some part in follicle activation after grafting of human ovarian tissue. Our group [3], as well as that of Meirow [4, 14], both demonstrated that primordial follicle activation occurs spontaneously shortly after transplantation and is responsible for follicle loss (burnout). This was proved by the upturn in the growing follicle population and proliferation of GCs in human ovarian tissue xenografts. However, two crucial questions emerged: (i) Is promotion of follicle activation in grafts via Akt stimulators actually necessary, as follicle activation happens spontaneously after transplantation? (ii) Since post-transplantation follicle activation (along with ischemia) could well cause follicle loss [4], should we not try (at the outset or at least very soon after grafting) to prevent this depletion by reducing activation rather than promoting it?
To address these two questions, two studies with human ovarian tissue were conducted by our team [15, 16].
First study
Protocol
Experimental design
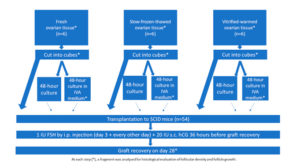
Human ovarian tissue was xenografted into 54 immunodeficient mice (Figure 1). The xenografting procedure has been extensively documented in an earlier publication [17]. Use of human ovarian tissue was approved by the Institutional Review Board of the Université Catholique de Louvain (2012/23MAR/125, N° B403201213872).
The grafting experiment was performed using fresh ovarian tissue (group I, n=6 women, mean age: 30 years, range: 26-33 years), slow-frozen-thawed ovarian tissue (group II, n=6 women, mean age: 20 years, range: 18-21 years), and vitrified-warmed ovarian tissue (group III, n=6 women, mean age: 29 years, range: 23-34 years). Fresh ovarian tissue pieces (measuring between 6x10x1 mm and 8x10x1 mm) were kept on ice in transport medium (Universal IVF Medium, Origio, Denmark), then immediately transferred from the operating room to the laboratory. For slow-freezing and thawing, standard protocols used in our institution to cryopreserve ovarian cortex from patients wishing to preserve their fertility were applied, as described in previous publications [18, 19]. Vitrification was achieved using the Ova Cryo Kit, type M (catalog VT301S, Kitazato BioPharma Co., Ltd. Fuji, Shizouka, Japan) and the Cryo Device, type M (catalog ODTx10, Kitazato BioPharma Co., Ltd. Fuji, Shizouka, Japan), following the manufacturer’s instructions. Tissue fragments were assigned either for immediate grafting, or for culture with or without IVA prior to transplantation (Figure 1).
Following a previously reported protocol [9], the animals were given an intraperitoneal injection of 1 IU human FSH (hGonal, Merck Serono, Germany) on the third post-transplantation day, which was continued every other day over the 4-week period [17]. Just 4 weeks post-reimplantation is likely too soon to detect preovulatory follicles in grafted human tissue; however, in order to mimic Kawamura’s protocol [9], 20 IU human chorionic gonadotropin (hCG, Pregnyl®, Organon, the Netherlands) was injected subcutaneously 36 hours before graft retrieval, and at 28 days, the mice were euthanized by cervical dislocation and the recovered grafts were fixed in 4% (v/v) formaldehyde.
Histological analysis
Surface measurements were calculated using the Mirax Viewer program (Zeiss, Jena, Germany). Follicles were counted and categorized according to Gougeon's classification into primordial, intermediate, primary, secondary or antral [20]. Only follicles with a clearly visible nucleus were evaluated. Atresia of early follicles was diagnosed according to the strict criteria of Gougeon and Chainy [21]. The prevalence of atretic follicles was ascertained [21], and follicular density was calculated as the total number of follicles/mm2.
Statistical analysis
For intragroup analyses of follicular density, results were compared using parametric paired t-tests and the non-parametric paired Wilcoxon test. For intergroup analyses, the non-parametric unpaired Mann-Whitney test was applied. One-way ANOVA was used for follicle staging. P-values <0.05 were considered significant.
Results and discussion
Eighteen human samples were included in the in vivo experiment (Figure 1). On day 28, all the grafts (100%) were clearly visible and successfully retrieved from all the groups.
Follicular density
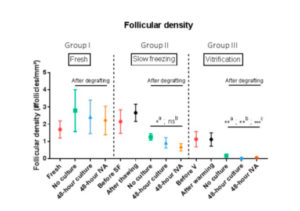
Follicular density can be seen in Figure 2. In a comparison of post-grafting follicular density between groups, the slow-freezing group (group II) did not show any significant difference in follicular density compared with the fresh tissue group (group I) (p=0.25, p=0.39 and p=0.25 for the no culture, 48-hour culture and 48-hour IVA subgroups respectively). On the other hand, all grafted tissues in the vitrification group (group III) exhibited a significant decline in follicular density compared with the fresh tissue (group I) (p=0.05, p<0.01 and p=0.02 for the no culture, 48-hour culture and 48-hour IVA subgroups respectively). The decrease in follicular density in group III was also significant (p<0.0001) compared with group II (slow-freezing), with mean density in grafted vitrified tissue reaching 0.04 follicles/mm2.
Numerous studies have investigated and compared slow-freezing-thawing and vitrification-warming procedures for ovarian tissue [22, 23], but there is a lack of consistent results in the literature. This may be due to heterogeneity of the cryopreservation protocols and cryoprotectant concentrations applied, the variety of animal models used, the adequacy (or otherwise) of graft site preparation, as well as the different grafting durations and/or methods employed to assess ovarian tissue quality.
In a previous study [15], we demonstrated that transplanting vitrified ovarian tissue immediately after warming causes a more significant decline in follicular density than grafting slow-frozen tissue. It appears that the combination of vitrification and grafting is deleterious to the post-grafting follicle reserve. Indeed, after comparing follicular density in the no culture, 48-hour culture and 48-hour IVA groups, we concluded that culturing ovarian tissue for 48 hours with PTEN inhibitors and PI3K stimulators prior to grafting is damaging to the follicle reserve after both slow-freezing and vitrification, but the decline in the ovarian stockpile is significantly more pronounced with the latter.
Effect of IVA culture before grafting on primordial follicles
Addition of PI3K stimulator and PTEN inhibitor for 48 hours in culture did not significantly change the proportion of primordial follicles in any of the groups (fresh, slow-frozen or vitrified tissue) compared to 48 hours of control culture without these molecule (15).
Growing follicles
The transplantation procedure yielded an increase in the percentage of growing follicles in fresh, slow-frozen-thawed and vitrified ovarian tissue after grafting, providing the ideal way to analyze the degree of activation. This is in line with our previous studies, in which both slow-frozen-thawed and vitrified-warmed human ovarian tissues were xenografted into nude mice [3, 23] and showed a high proportion of growing follicles, proving follicle activation. This activation, followed by atresia, is what causes the dramatic decline in follicular density [3], described as burnout of the follicle pool [4, 14]. The challenge was to establish whether the IVA protocol could influence follicle maturation.
Conclusions of study 1
By evaluating percentages of growing follicles after culture with PTEN inhibitors and PI3K activators, and comparing them with the group cultured for 48 hours without Akt stimulators, we found that the numbers of growing follicles in grafts from groups I (fresh) and II (slow-frozen) were not affected by culture with IVA. Despite our attempts, we were unable to reproduce the results of Kawamura et al. [9] by replicating their experimental model. Moreover, our study appears to suggest that the technique may actually be deleterious to the ovarian reserve, as massive follicle activation could lead to large-scale atresia. In an opinion paper, Meirow et al. cast doubt on the efficacy of the IVA technique in clinical practice [24].
Recently, Kawashima and Kawamura [25] described a ‘novel’ infertility treatment based on mechanobiology, where they effectively abandoned the vitrification step and IVA with PTEN inhibitors and Akt stimulators. Their so-called IVA approach was limited to fragmenting ovarian cortex into small cubes (1-2 mm). However, we would like to point out that it is known from the literature [3, 24], as well as the present study, that activation of primordial follicles and growth to the secondary stage occur after grafting irrespective of the tissue used, be it fresh, slow-frozen or vitrified, and with or without culture with PTEN inhibitors and Akt stimulators. Furthermore, fragmentation into very small pieces followed by transplantation is possibly not the best approach. Meirow et al. [24] did not observe any advantage of transplanting small fragments rather than larger pieces. In fact, no ovarian activity or follicle growth were detected inside the ovary where the small fragments had been implanted.
We were not able to demonstrate any significant benefits of IVA in human ovarian tissue xenografted into immunodeficient mice, and therefore consider it unlikely that IVA enhances follicle viability. We feel strongly that both IVA and vitrification need to be thoroughly investigated before contemplating their application in a clinical context. Kawamura himself appears to have abandoned his former technique of promoting follicle activation with PTEN inhibitors and PI3K stimulators, and now limits the practice to fragmenting only fresh ovarian tissue prior to its reimplantation.
Second study
The second study was conducted to shed light on follicle dynamics in the early days after grafting of frozen-thawed human ovarian tissue, and the role of the Akt pathway in transplantation-induced follicle activation.
Experimental design
Frozen-thawed human ovarian tissue from 6 different patients (mean age: 29.8 years, range: 25-35 years) was used for the study after obtaining Institutional Review Board approval (2012/23MAR/125). Four fragments measuring approximately 5×8×1.5 mm³ were taken from each patient, one for control purposes and three for transplantation. Three grafting durations were determined: 3, 7 and 21 days.
Results and discussion
Follicle density, classification, growth and atresia
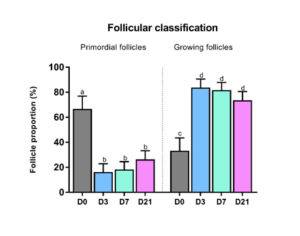
A significant decrease was observed both in follicular density (p=0.04) and in the proportion of primordial follicles (Figure 3) present in the three grafted groups compared to non-grafted tissue. Indeed, the percentage of primordial follicles in non-grafted tissue (66.7%) dropped significantly after grafting: 16.2% on day 3 (p=0.01), 18.3% on day 7 (p=0.007) and 36.3% on day 21 (p=0.02). Conversely, growing follicles showed a significant increase in number after 3 days (83.8%, p=0.01). There was a significant upturn in rates of Ki67-positive primordial follicles in all groups after grafting [16].
Follicle activation was evaluated by immunostaining for phospho-Akt (p-Akt). Slides were incubated overnight at 4°C with primary antibody, namely rabbit anti-human p-Akt (Ser473) (dilution 1/50, #4060; Cell Signaling Technology, Danvers, MA, USA). Both positive and negative controls were conducted. Only follicles in the central area with a visible oocyte were analyzed. After delineation and annotation, computer-assisted quantification of staining concentrations was achieved with Leica's proprietary artificial intelligence (Tissue IA; Wetzlar, Germany).
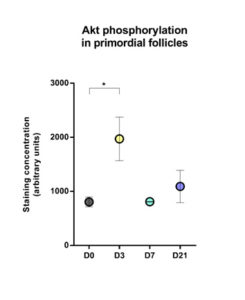
Significantly increased Akt phosphorylation was observed in primordial follicles after 3 days of grafting compared to non-grafted tissue (mean staining concentration expressed as arbitrary units: 1970 on day 3 versus 805.1 in non-grafted tissue, p=0.048; Figure 4).
The aim of this study was to elucidate the phenomenon of transplantation-induced follicle activation and the possible role of the Akt pathway. On day 3 post-transplantation, a significant decrease was detected in primordial follicle numbers, accompanied by a significant increase in the proportion of growing follicles. These data are consistent with previous results published by this and other teams on premature follicle activation, otherwise known as the burnout effect, which is characterized by large-scale activation of quiescent follicles resulting in uncontrolled growth and inevitable depletion of the ovarian reserve.
On the basis of these results, it is thought that a dual response is triggered when ovarian tissue is grafted, involving follicle death, but also accelerated growth of primordial follicles to more advanced stages. It is postulated that the Akt pathway could well be implicated in these events. Akt phosphorylation was higher in primordial follicles after 3 days of grafting than in controls. The vital role of the PI3K/Akt pathway in primordial follicle activation and ovarian stockpile maintenance has already been widely documented in mice, but this study has allowed us to confirm its role in humans, too. Indeed, the immediate post-transplantation period is characterized by hypoxia, and the cellular response to ischemia is bimodal, causing either cell death or activation of cell survival mechanisms, including the PI3K/Akt pathway. Hence, the increase in Akt phosphorylation observed in our recent study [16] is a direct consequence of severe hypoxia on day 3 post-grafting. Akt phosphorylation then returns to normal levels as functional vessels appear and physiological oxygen concentrations are restored [26].
General conclusions
In conclusion, our studies confirm that activation of primordial follicles and their growth to later developmental stages are early events that occur in human ovarian tissue after transplantation. Increased Akt phosphorylation may explain this early activation after grafting, contributing to premature depletion of the ovarian reserve, namely follicle burnout.
AUTHOR CONTRIBUTION
MMD and RM contributed both to the experimental design, experimental procedures and manuscript redaction.
FUNDING
This study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (FNRS grant no 5/4/150/5 awarded to Marie-Madeleine Dolmans and grant Télévie 7.4590.16 to Rossella Masciangelo), Fonds Speciaux de Recherche and the Foundation Against Cancer.
ACKNOWLEDGMENTS
The authors thank Mira Hryniuk, BA, for reviewing the English language of the manuscript and Deborah Godefroidt for her administrative help.
CONFLICT OF INTEREST
The authors declare no conflict of interest.