Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of childbearing age 1. The features of the disorder are anovulatory cycles, hyperandrogenism, and a specific ovarian appearance, characterized by an increased number of follicles and a greater ovarian volume 2. In addition to infertility, PCOS is also associated with an increased risk of metabolic disturbances such as obesity and hyperinsulinemia 3.
Lately, a possible link between hepatic steatosis, also called non-alcoholic fatty liver disease (NAFLD), and PCOS has attracted increasing attention 4-6. It is important to detect cases of NAFLD, since it may develop into non-alcoholic steatohepatitis, leading to an increased risk of cirrhosis and hepatocellular carcinoma 7. Recently, a UK-based cohort study involving 184,274 women found that the rate of NAFLD was about two times higher in women with PCOS than in controls (hazard ratio 2.23, 95%CI 1.86-2.66) 8. This study is the largest epidemiological study of this topic, and its results are in agreement with previous reports from smaller cohorts and case-control studies 6.
Despite coherent results from epidemiological studies, the association between PCOS and NAFLD still raises questions. First, the diagnostic modality used in different studies may produce varying results. Ultrasonography and computed tomography have been used previously, but both are poor modalities for reliably detecting NAFLD 9. It is therefore important to evaluate new and more precise techniques able to identify NAFLD in PCOS. Magnetic resonance imaging (MRI) is considered the most accurate modality for assessing hepatic steatosis, especially in the presence of low hepatic fat infiltration. Quantifying hepatic fat using an MRI Dixon technique to estimate the proton-density fat fraction (PDFF) is as easy, fast and accurate as using MRI spectroscopy, and even more precise than histologic grading 10,11.
Second, an important question that remains to be addressed is whether NAFLD in women with PCOS may be improved by lifestyle intervention as first-line treatment. Although the effect of lifestyle intervention on NAFLD in non-PCOS individuals has been addressed in several studies giving variable results, a recent meta-analysis suggested that exercise alone or in combination with dietary intervention may improve serum levels of liver enzymes and levels of hepatic fat 12. However, it is not known whether similar interventions may contribute to improvements in NAFLD in women with PCOS, too. This aspect is important to evaluate as recent reports have suggested that NAFLD is associated not only with obesity in PCOS, but also with endocrine disturbances such as hyperandrogenism and menstrual disorders.
The primary aim of this study, a sub-study of a randomized controlled trial (RCT), was to use the most precise technique, i.e. MRI, to evaluate hepatic fat concentration and the frequency of steatosis in overweight/obese women with PCOS before and after a behavioral modification intervention. The secondary aim was to investigate hepatic fat concentration in relation to endocrine and metabolic factors in these women.
Methods
The study was approved by the regional ethics committee, and informed consent was given by all the subjects (2012/146-31/3). The clinical trial registry number was ISRCTN48947168.
Study design and participants
This is a sub-study of an RCT investigating the effects of behavioral modification in 68 overweight/obese women with PCOS 13. Briefly, women fulfilling all three Rotterdam criteria for PCOS, aged 18-40 years, with a body mass index (BMI) ≥ 27 kg/m2 were allocated to receive behavioral modification intervention or minimal intervention in a 1:1 ratio for a period of four months with a follow-up after 12 months. The behavioral modification intervention consisted of a course with group meetings three times per month during the intervention period. The course, led by a lifestyle coach, was based on various elements: up-to-date knowledge concerning weight control, personal leadership and mindfulness, as well as physical activity and diet. In addition, the participants received individual coaching sessions once a month with the course leader. Participants allocated to minimal intervention instead received general healthy lifestyle recommendations given by a midwife, supported by a pamphlet with written advice about diet and exercise. A detailed description of the study design and interventions has been published previously 13.
From the RCT population, 22 participants (11 active treatment and 11 control treatment) were randomly selected to undergo MRI investigations before and after the intervention in order to quantify hepatic fat concentration.
MRI sequences
A Siemens Verio 3T system was used for estimation of PDFF acquired using a breath-hold (15 seconds) two-dimensional gradient multi-echo sequence with five echoes. Due to the participants’ large waist circumferences, two body coils were used in parallel to cover the upper abdomen. Relevant scan parameters were: echo time 1/Δ echo time/ repetition time = 1.05 ms/1.73 ms/214 ms, flip angle = 13°, matrix = 192 × 156, field of view = 480 × 390 mm, voxel size = 2.5 × 2.5 × 10 mm3, slice spacing = 0 mm, Nslices = 13, acceleration factor = 2 (GRAPPA), and number of excitations = 2. Two acquisitions were used to cover the entire liver in the feet-head direction. A Dixon-based fat-water separation was used to calculate fat fraction maps using an iterative graph-cut algorithm 14.
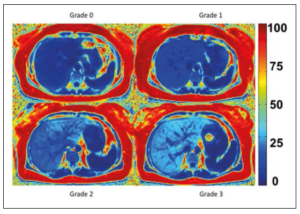
A radiologist (M.S.) delineated the whole liver, excluding the vena cava inferior, on fat fraction maps, and mean PDFF with standard deviation was calculated. In accordance with Tang et al., hepatic steatosis grade 1 was defined as PDFF = 6.4-17.3%, grade 2 as PDFF = 17.4-22.0%, and grade 3 as PDFF ≥ 22.1% 15. Representative PDFF-MRI images of the different grades are given in Figure 1.
Biochemical analysis
Serum levels of steroid hormones were determined by liquid chromatography-tandem mass spectrometry at the Endoceutics Laboratory, Quebec, Canada, as previously described 16,17. Luteinizing hormone (LH), follicle-stimulating hormone (FSH), and sex hormone-binding globulin (SHBG) were analyzed using electrochemiluminiscence immunoassay from Roche Diagnostics AG (CH 6343 Rotkreuz, Switzerland) (Cobas8000®). Anti-Müllerian hormone (AMH), lipids (cholesterol, HDL, LDL, triglycerides) and liver enzymes (AST, ALT) were analyzed according to manufacturers’ protocol at the Department of Clinical Chemistry, Karolinska University Hospital, Stockholm, Sweden.
Free androgen index (FAI) was calculated (testosterone nmol/L divided by SHBG nmol/L × 100) and ≤ 5 was regarded as normal. The homeostatic model assessment to quantify insulin resistance (HOMA-IR) was calculated as (glucose × insulin) / 22.5, and a value below 2.0 was regarded as normal.
Statistics
Data are presented as means ± SD or as percentages. The Mann-Whitney test was used for between groups comparisons of continuous variables, and the Spearman correlation test was used to test correlations. The Wilcoxon signed ranks test was used to compare continuous variables before and after intervention. Fischer’s exact test was used to compare frequencies. All tests were two-sided and performed at the 5% significance level.
Results
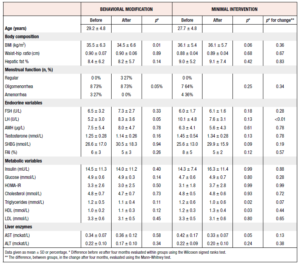
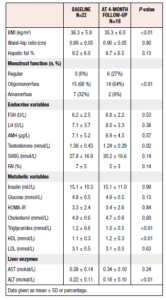
A total of 22 women entered the study and underwent MRI examination at baseline, 11 being allocated to behavioral modification intervention and 11 to minimal intervention. There were no differences in baseline characteristics except for LH, which was significantly higher among participants randomized to the control group (Table 1). 18 women completed the 4-month study period and underwent a second MRI examination.
Hepatic fat at baseline
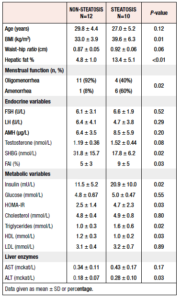
At baseline, 10 out of all 22 (45%) participants had hepatic steatosis, i.e. a PDFF ≥6.4%, in seven cases classed as grade 1, in two as grade 2, and in one as grade 3. These women with hepatic steatosis had higher BMI, FAI, fasting insulin, HOMA-IR, triglyceride and ALT values than those without increased hepatic fat. Furthermore, they had a higher frequency of amenorrhea and lower SHBG and HDL values than those without hepatic steatosis (Table 2).
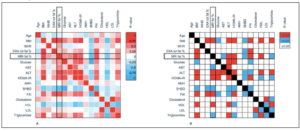
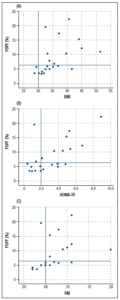
To provide an overview of the baseline factors that correlated with hepatic fat, we constructed a correlation matrix (Figure 2). This showed that hepatic fat correlated positively and significantly with BMI, AST, ALT, HOMA-IR, FAI, and triglycerides. In addition, hepatic fat correlated negatively with both SHBG and HDL.
In more detail, BMI ranged between 29 and 51 kg/m2 and strongly correlated positively with hepatic fat, R = 0.68, p = 0.001 (Figure 3A). All participants with hepatic steatosis had a BMI ≥ 32 kg/m2. The highest BMI recorded without concomitant hepatic steatosis was 42 kg/m2. HOMA-IR also strongly correlated positively with hepatic fat R = 0.62, p = 0.002 (Figure 3B). Furthermore, FAI correlated positively with hepatic fat R = 0.66, p = 0.001 (Figure 3C) and mean FAI was higher in women with hepatic steatosis than in women without steatosis (Table 2).
Taken together, the results from the baseline investigations showed that women with PCOS and increased hepatic fat had more severe endocrine, metabolic and hepatic disturbances, as well as higher frequency of amenorrhea, than PCOS women without increased hepatic fat.
Hepatic fat after four-month intervention
A total of 18 participants completed the 4-month intervention and underwent a second MRI examination (8 in the behavioral modification intervention group and 10 in the minimal intervention group).
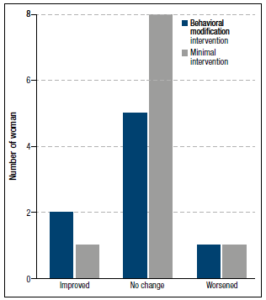
After the intervention, there was no significant difference between the randomized groups in the proportions of women who had improved, worsened or remained within the same grade (Figure 4). In three participants, hepatic fat concentration was downgraded after four months as compared to baseline, in two it was upgraded, and in the remaining 13 it remained stationary. Furthermore, there was no difference in endocrine or metabolic factors between the randomized groups after four months (Table 1). Only the change in LH, with women randomized to behavioral modification showing increased levels and those randomized to the control treatment recording decreased levels, was significantly different.
The whole study cohort showed a minimal but significant weight loss post intervention (100.8 ± 18.1 vs 98.0 ± 18.2, p=0.02) (Table 3). Moreover, eight of the 18 participants (44%) showed improved menstrual function, defined as going from amenorrhea to oligomenorrhea or regular cycles, or from oligomenorrhea to regular cycles. Serum levels of testosterone also decreased significantly. The frequency of hepatic steatosis in the whole study cohort was unchanged (44%) after four months and the mean hepatic fat % was not significantly changed (Table 3). However, triglycerides and ALT decreased, whereas HDL increased after the intervention.
To investigate what factors correlated with the change in hepatic fat concentration we performed a correlation matrix. We found that only the change in fasting glucose correlated with the change in hepatic fat (R=0.55, p = 0.03); no other endocrine or metabolic changes correlated with the change in hepatic fat (data not shown). The small number of participants did not allow us to perform a stepwise multi-logistic regression analysis to analyze predictors in a controlled fashion.
Discussion
This is the first study in obese women with PCOS to use MRI to evaluate the change in hepatic fat after vs before a behavioral modification intervention. We found that 45% of obese women with PCOS had hepatic steatosis at baseline and that these women had higher BMI, FAI, fasting insulin, HOMA-IR, triglyceride and ALT values, and lower SHBG than those without steatosis. They also had a higher frequency of amenorrhea. Despite significant weight loss in the whole study population and improved menstrual function after four months of lifestyle changes, there was no significant decrease in hepatic fat concentration.
A retrospective study using ultrasound detected hepatic steatosis in 55% of PCOS patients, compared with 39% of lean women (BMI < 25 kg/m2) 18, while another study found no hepatic steatosis in lean women 19. These results are in line with an association between hepatic steatosis, insulin resistance and obesity as shown in ultrasound-based studies 18,20. In our study, lean PCOS patients were not included, but interestingly none of the participants with BMI < 32 kg/m2 had hepatic steatosis.
There are few studies investigating liver fat in PCOS using MRI, which is considered the most accurate method. Jones et al. used MRI spectroscopy and suggested that hepatic steatosis in PCOS is independent of insulin resistance and obesity. Mean liver fat in their 29 patients with PCOS was 6.1% (95%CI, 2.6–12.7) 21. A second study by Cree-Green et al. showed, as in other studies, a prevalence of approximately 50% hepatic steatosis in PCOS. However, the MRI technique used was not described and the liver fat content was low, with a mean value of 5.6% in the PCOS group and 2.2% in the control group 22. A third study showed a mean liver fat content of 10.2% (SD 1.1%) at study entry in 25 patients with PCOS; after intervention with omega-3 fatty acid supplementation, liver fat content decreased significantly compared to placebo 23. In our study, we were able to classify low grades of hepatic steatosis by MRI, which is difficult using computed tomography or ultrasound. Contrary to the study by Jones et al., we found significant correlations at baseline between hepatic fat and BMI, hepatic fat and HOMA-IR, as well as between BMI and HOMA-IR. Since no participant had hepatic steatosis if their BMI was less than 32 kg/m2, while HOMA-IR could be pathological without hepatic steatosis, the strongest link to hepatic steatosis appears to be BMI.
A study using computed tomography for liver fat estimation found, on multiple regression analysis, that total testosterone made a large contribution and suggested that liver fat in PCOS was associated with hyperandrogenism 24. A systematic review and meta-analysis concluded that hepatic steatosis is associated not only with obesity and insulin resistance, but also with hyperandrogenism. However, none of the included studies used MRI for liver fat estimation 6. Another study using ultrasound found an independent association with hyperandrogenism and hepatic steatosis in PCOS 25. In our study, we also found a correlation between hepatic steatosis and hyperandrogenism measured as FAI. After BMI, this was the second strongest correlation with hepatic fat percentage. It has previously been reported that SHBG decreases with hepatic steatosis, which will increase FAI 26. However, 50% of participants with elevated FAI in their study did not have hepatic steatosis 26. Our results contribute to these findings with the use of MRI, which shows much higher precision than the ultrasound performed in previous studies. We found that two-thirds of those with increased FAI had steatosis. Furthermore, previous studies on liver fat in PCOS have not been able to grade steatosis, which was possible in this study.
After four months of intervention, we did not notice a difference in improvement of hepatic fat percentage between the women randomized to behavioral modification intervention and those receiving minimal intervention. Furthermore, there was no change in hepatic fat in the whole study population, even though there was a significant weight loss after intervention. Hence, there was no correlation between weight loss and change in hepatic steatosis. We interpret this as an effect of the short treatment period (four months), as well as the limited weight loss obtained (mean -2.8 kg). However, it cannot be ruled out that the lack of improvement in hepatic fat is explained by the low number of participants in the study.
Interestingly, however, menstrual function improved in 8/18 patients, indicating that reproductive function improves much more easily than hepatic fat proportion. The results also suggest that improvement in menstrual function in obese women with PCOS is not dependent on improvement in hepatic fat. The only correlation we found was a positive one between the change in fasting glucose and the change in hepatic fat. This finding supports an association between hepatic steatosis and glucose metabolism.
ALT levels also decreased, and blood lipids improved after four months. As expected, AST and ALT were poor predictors of hepatic steatosis, as only two patients had pathological AST at baseline (none had pathological ALT) with only one of these having hepatic steatosis.
Since this is a sub-study of a larger RCT, only a limited number of patients underwent MRI. Despite this relatively low number of patients, we were able to demonstrate differences between obese women with PCOS who had hepatic steatosis and those who did not. However, the study sample was too small to allow us to perform stepwise logistic regression analysis to identify predictors of hepatic steatosis and we also cannot rule out that the absence of improvement in hepatic steatosis was due to the small sample size. Furthermore, the intervention period was relatively short, and we were not able to perform another MRI at 12 months’ follow-up. A further limitation of this study is that there was no control group of obese women without PCOS. However, the study, being a randomized interventional study, was not designed to test a control group of women without PCOS. In addition, no patient with a lean BMI was included, although in our study no patient with BMI < 32 kg/m2 had hepatic steatosis.
The strengths of the study include the randomized design and the assessment of hepatic fat by MRI. Performing MRI on patients with large waist circumference requiring large fields of view, as in our study, is problematic due to magnetic field inhomogeneity in the periphery of the gantry. In these cases, the Dixon technique combined with an iterative graph-cut algorithm is well suited for fat estimation.
In conclusion, hepatic steatosis appears to be common in obese women with PCOS. It seems to be associated not only with BMI, but also low with SHBG, hyperandrogenism and insulin resistance. The behavioral modification intervention used in this study did not significantly improve hepatic steatosis over the four months of the study period. Since hepatic steatosis is relatively common in PCOS, PDFF-MRI would be indicated in obese PCOS patients, although this is not yet a method routinely used in clinical practice. Due to their higher risk of developing non-alcoholic steatohepatitis, further studies of the effect of lifestyle intervention and other interventions on hepatic steatosis in obese women with PCOS are warranted.
Conflicts of Interest Statement:
The authors have no conflicting interests to declare.
Financial Support
This work was supported by the Swedish Medical Research Council (ALH 2017-02051) and the Stockholm County Council (ALH 2016-0349).