Introduction
Polycystic ovary syndrome (PCOS) is a frequent disease that impairs reproductive function; it occurs in about 4-25% of women of reproductive age (1, 2). Its diagnostic criteria were established at the American Society for Reproductive Medicine and European Society for Human Reproduction and Embryology consensus meeting in Rotterdam (3). Classically the diagnostic criteria require patients to show at least two of the following three features: i) chronic anovulation disorder (oligo or anovulation leading to amenorrhea); ii) clinical (acne, hirsutism) or biochemical signs of hyperandrogenism; iii) the presence of micro-polycystic ovaries at ultrasound or the presence of 12 or more follicles with a diameter of 2–9 mm in each ovary, and/or increased ovarian volume (> 10 ml).
Over the past decade the dysmetabolic state of insulin resistance (IR) and its correlate, compensatory hyperinsulinemia, have been considered important additional aspects. Since IR and compensatory hyperinsulinemia have been considered frequently occurring features of the syndrome, it is now widely recognized that, in addressing and treating the various clinical aspects of PCOS, IR is a problem that needs to be evaluated and solved (4). Apart from the use of oral contraceptives and/or various anti-androgenic drugs in PCOS, there is extensive literature evidence of positive effects when using specific drugs to counteract IR, such as metformin (4, 5), or when administering complementary substances, such as inositols, alpha lipoic acid, or combinations of these (6-11). Other complementary compounds, such as carnitines (12), L-arginine (L-ARG) and N-acetyl-cysteine (NAC) (13), have also been reported to counteract compensatory hyperinsulinemia. All these have been demonstrated to be effective on IR and on PCOS features, specifically when administered at high daily dosages, such as 2-3 gr/day for carnitines (12), 1.6 gr/day for L-ARG, and 1.2 gr/day for NAC (13). They are all effective, both alone and in combination with other drugs such as metformin, clomiphene citrate or gonadotropins, improving metabolism, reproductive functions, ovulation rate and pregnancy in PCOS patients (14-19).
On this basis we to evaluate the possible impact of long-term administration (6 months) of a combination of low doses of carnitines, L-ARG and NAC on hormonal and metabolic parameters and on the hepatic insulin extraction index (HIE) in a group of obese PCOS patients.
Materials and Methods
Subjects
Between January 2016 and December 2018, a total of 53 overweight/obese PCOS patients gave their informed consent to participate in the present study. All were attending the outpatients’ clinic of either the Gynecological Endocrinology Center at the University of Poznan, Poland or the Gynecological Endocrinology Center at the University of Modena and Reggio Emilia, Italy. All these patients were complaining of menstrual irregularities and/or PCOS and had requested not to be treated with oral contraceptives; for this reason, an integrative/complementary treatment was proposed.
All the patients fulfilled the criteria established by the American Society for Reproductive Medicine and the European Society for Human Reproduction and Embryology for diagnosing the presence of PCOS (20), and at least two of the following criteria had to be present: (a) oligomenorrhea with inter-menstrual intervals longer than 45 days, (b) clinical (acne, hirsutism) or biochemical signs of hyperandrogenism, (c) the presence of micro-polycystic ovaries at ultrasound.
In addition, patients had to fulfil the following criteria: (d) absence of enzymatic adrenal deficiency and/or other endocrine disease, including diabetes, (e) normal prolactin (PRL) levels (range 5–25 ng/ml), (f) no hormonal treatment during a period of at least 6 months prior to the study, (g) body mass index (BMI) above 26. None of the subjects enrolled had taken medications and/or steroids, oral contraceptives or metformin within the 3 months prior to the evaluation.
The complementary treatment was given as sachets containing acetyl-L-carnitine (250 mg), L-carnitine (500 mg), L-ARG (500 mg), NAC (50 mg) and minimal amounts of Vit E, Vit C, Vit B6 and Vit B12 (Proxeed Women, The Netherlands). No lifestyle or dietary changes were required of the patients, and all were studied, the first time, on day 3–6 of the menstrual cycle, if present. The post-treatment follow-up was performed after 12 and 24 weeks of treatment, plus a few days if necessary, so that patients were again evaluated on day 3–6 of the menstrual cycle (the first occurring after the 12 or 24 weeks of treatment). Of the total 53 PCOS patients who agreed to participate in the study, only 45 completed the 24 weeks as per the study design; the other 8 patients (8 out of 53, 15.1%) were found to be pregnant during the first 6-12 weeks of supplementation. For this reason, they suspended the treatment and were excluded from the study.
All the remaining patients (n=45) were evaluated for luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2), progesterone (P), androstenedione (A), testosterone (T), insulin, C peptide, total cholesterol, HDL, LDL, glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) concentrations. The HOMA index was computed to estimate sensitivity to insulin (20).
An oral glucose tolerance test (OGTT) was performed to measure insulin, C peptide and glucose levels, with sampling taking place before and 30, 60, 90, 120, 180 and 240 min after oral assumption of 75 g of glucose. This was done both before and after 12 and 24 weeks of integrative treatment. A hyperinsulinemic response is recognized when insulin plasma levels are above 50 μU/ml within 90 min of glucose load at time 0 (19).
According to this criterion, 39 of the 45 patients (86.6%) were found to be hyperinsulinemic and 6 (13.4%) normoinsulinemic.
Assay
All samples from each subject were assayed in the same assay. Plasma LH and FSH concentrations were determined using a previously described immunofluorometric assay (21, 22). The sensitivity of the assay, expressed as the minimal detectable dose, was 0.1 IU/ml. The cross-reactivities with free and ß-subunits of LH, FSH and thyroid-stimulating hormone (TSH) were less than 2% (20). Intra-assay and inter-assay coefficients of variation were 4.3% and 6.5%, respectively. Plasma C peptide, E2, A, cortisol and T levels were determined by commercially available assay systems. Based on two quality control samples, the average within- and between-assay coefficients of variation were 3.5% and 8.4%.
Plasma insulin and C peptide concentrations were determined using an immunoradiometric assay (Biosource Europa S.A., Nivelles, Belgium). Based on two quality control samples, the average within- and between-assay coefficients of variation were 4.0% and 10.2%.
Statistical analysis
After analysis of variance (one-way ANOVA), data were tested for statistically significant differences between the groups (before and after 12 and 24 weeks of the treatment) by means of Student’s t-test for paired and unpaired data, as appropriate.
The HOMA index was computed to estimate sensitivity to insulin (20) since it is considered the main index of the insulin resistance and metabolic syndrome, and a common link between the coexisting abnormalities; it can be calculated by homeostasis model assessment of IR (HOMA-IR) as (fasting insulin mU/l) × (fasting glucose mmol/l)/22.5 (20). We used the previously established cutoff value of 2.7 (20).
The HIE was computed as the ratio between insulin and C peptide plasma concentrations (insulin/C peptide) (23). The area under the curve of the HIE under the OGTT glucose load (AUC, subtracted from the baseline value) was computed using the trapezoid formula.
Data are expressed as mean+SEM.
Results
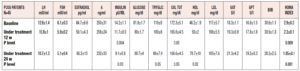
The patients’ hormonal and metabolic parameters are reported in Table 1. Significant improvements in some of the metabolic parameters studied, namely total cholesterol, HDL, triglycerides, plasma insulin levels and HOMA index, were observed after 12 and also after 24 weeks in the entire group of patients (n=45). No changes were observed in the levels of any of the reproductive hormones (Table 1).
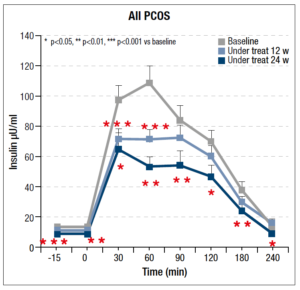
As regards the OGTT, significant changes were recorded throughout the 24 weeks of integrative treatment with the insulin response to the glucose load found to decrease significantly, as can be seen in Fig. 1; the maximal response reduction, of almost 50%, occurred within 60 minutes.
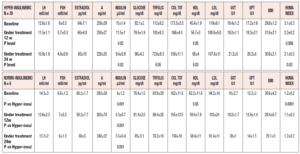
When the patients were subdivided according to their insulin response to the OGTT, specific differences were observed between those with a norminsulinemic and those with a hyperinsulinemic response (Table 2). The most notable finding was that 39 of the 45 (86.6%) PCOS patients were found to be hyperinsulinemic, while only 6 (13.4%) were normoinsulinemic, despite being overweight/obese (Table 2).
This subdivision revealed a number of marked differences between the two groups (Table 2). In fact, while they did not differ in reproductive hormonal and some metabolic parameters, the two groups showed considerable differences in insulin and HDL plasma levels as well as HOMA index (Table 2). Interestingly, the normoinsulinemic group showed absolutely normal insulin, HDL and HOMA index values.
When data from the two groups were evaluated after 12 and 24 weeks of complementary treatment, no changes were observed in the normoinsulinemic group, while significant changes were recorded in the hyperinsulinemic patients (Table 2). In fact, this latter group showed progressive improvements in insulinemia, plasma HDL and HOMA index values throughout the 24 weeks of the treatment.
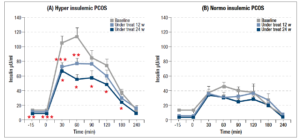
It should be noted that these parameters remained significantly different from what was observed in normoinsulinemic subjects (Table 2). Furthermore, the two subgroups also differed markedly in their insulin response to OGTT (Fig 2, panels A and B). In fact, plasma insulin levels were significantly reduced in hyperinsulinemic subjects (Fig. 2, panel A) after 12 and 24 weeks of treatment compared with baseline, and the maximal insulin response reduction under glucose load brought the insulin levels down to values very close to normal (i.e. around 50 µU/ml). This change in the insulin response to the glucose load was also confirmed by the significant reduction of the AUC of insulin (p<0.01, data not shown).
On the contrary, no changes were observed in the insulin response in the normoinsulinemic PCOS group, which showed a perfectly normal insulin response throughout the 24 weeks of treatment (Fig. 2, panel B). The AUCs of insulin recorded by the hyperinsulinemic patients were significantly different at baseline, and after 12 and 24 weeks of treatment (p<0.006, p<0.05 and p<0.04 respectively) compared with those of the normoinsulinemic patients (data not shown).
To better understand the changes in and dynamics of compensatory hyperinsulinemia observed under the complementary treatment, we computed the HIE (23) at each OGTT sampling time, and then considered the area under the HIE curve (AUC HIE). The ratio between insulin and C peptide concentrations was thus computed for each sampling time. Since HIE is a specific index of hepatic insulin clearance/extraction by the liver, any changes observed might permit specific clinical considerations.
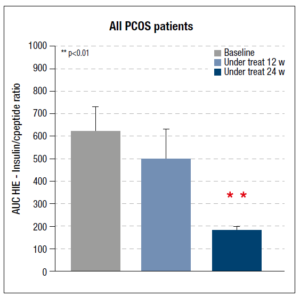
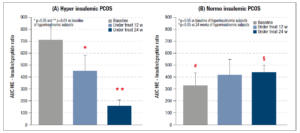
Considering the data for the whole set of PCOS patients, the AUC HIE decreased after 12 weeks but the reduction was significant only after 24 weeks of integrative treatment (Fig. 3). The same parameter was also computed for each of the two subgroups of PCOS patients (hyper and normoinsulinemic): the hyperinsulinemic patients showed a significant reduction after 12 and 24 weeks of treatment (Fig. 4, panel A). No changes were observed in the normoinsulinemic subjects (Fig. 4, panel B) who, however, at baseline had a significantly lower AUC HIE than the hyperinsulinemic subjects (Fig. 4, panel B), which remained unchanged throughout the treatment and at 24 weeks was higher than the value recorded in hyperinsulinemic subjects (Fig.4).
The patients’ menstrual diaries showed that in baseline conditions there were 35 oligomenorrheic and 10 eumenorrheic patients; after 24 weeks of integrative treatment eumenorrhea was reported in 34 subjects and 11 were oligomenorrheic (only 5 of these were still oligomenorrheic, as before the study).
Discussion
The present study reported that the use of a combination of nutraceutical compounds, specifically acetyl-L-carnitine, L-carnitine, L-ARG and NAC, was effective in reducing IR and in improving insulin sensitivity and HIE in obese PCOS patients.
Insulin resistance is a common feature of obese PCOS patients although not exclusive to this population. Over the past decade or so, numerous studies have shown positive effects, on IR, of several insulin sensitizers such as metformin (5), as well as of several complementary substances such as myo-inositol (6, 19), d-chiro inositol (7), alpha lipoic acid (24), or combinations of these (9, 10).
The neuroendocrinology of PCOS is quite complex (25), considering the relevant influences of genetic and of epigenetic factors on the occurrence of IR (26); therefore, it has been proposed to combine myo-inositol with d-chiro inositol, or each of these two with alpha lipoic acid (6, 7, 9, 10, 19, 27). These different complementary interventions were reported to be successful, with the dysmetabolic impairment found to be significantly reduced (6, 7, 9, 10, 19, 27).
Like inositols, carnitines (12, 28, 29), L-ARG and NAC (13, 30, 31) have also been reported to be effective on IR. All of them were demonstrated to decrease IR at quite high dosages (12, 30, 31), also in PCOS patients undergoing IVF programs (32). Moreover, PCOS patients, compared with healthy controls, have been demonstrated to have a higher grade of oxidation and lower nitric oxide levels (33), as well as significantly lower plasma carnitine levels (34).
Our data showed that a mix of these complementary substances was greatly effective in improving metabolic parameters. In fact, insulin sensitivity, lipid parameters and HOMA index were greatly improved despite the fact that no changes in lifestyle were requested. On the contrary, no changes in reproductive hormones were observed. The amounts of L-carnitine, acetyl-L-carnitine, L-ARG and NAC that we used (only 500 mg, 250 mg, 500 mg and 50 mg, respectively) were lower than those previously reported to be effective in PCOS patients (12, 30-32). Our data showed that a combination of lower amounts of these four complementary substances was able to modulate and reduce the insulin response to the OGTT and to improve some aspects of the lipid profile (increasing HDL and reducing total cholesterol), similarly to what has previously been reported for each of them singly (12, 28-31).
When the PCOS patients were subdivided by insulin response to the glucose load, as previously reported (19), two types of PCOS patients were disclosed: those with normal and those with hyperinsulinemic response to OGTT. In our population, the latter group was more represented, thus confirming previous evidence that not all obese PCOS are similar in terms of response to glucose load (19).
In the presence of a hyperinsulinemic response, which occurred in 39 out of our 45 PCOS patients (86.6%), the mix of complementary substances used was able to modulate the metabolic profile and decrease the excess of insulin, as well as improve the HOMA index and lipid concentrations during the entire 6 months of treatment. Interestingly, this integrative treatment did not modulate any one of the reproductive hormones, similarly to what has recently been reported using alpha lipoic acid (24). These results suggest that although insulin is able to act centrally, modulating the reproductive center (i.e. the KYDN neurons and then GnRH-secreting neurons) (25), the positive improvement of the metabolic impairment is probably not the only one needed to re-activate a normal reproductive profile, given that no significant changes in plasma LH concentrations were observed during the 6 months of treatment. Although we were not able to evaluate leptin and adiponectin in the present study it cannot be excluded that the lack of BMI changes in our patients might be an index of a lack of improvement of hormonal signals from fat tissues (i.e. adiponektin and leptin), which might better modulate the reproductive axis. Nevertheless, a high percentage of hyperinsulinemic subjects recovered normal menstrual cyclicity and only a few of them reported oligomenorrhea at the end of the observation period.
As regards the insulin response to the OGTT, the integrative treatment greatly decreased this insulin response in the whole group of PCOS patients. The hyperinsulinemic PCOS women showed the highest improvement, as expected, obtaining an up to 50% reduction of the insulin response after 6 months of treatment, with no changes in BMI. The few patients with a normoinsulinemic response to the OGTT did not show any change in insulin response to glucose load at any point during the treatment period. These findings support our previous observation (19) that not all obese PCOS patients show similar insulin dynamics under OGTT. In addition, these data suggest that the presence of an abnormal insulin response to glucose load is greatly related to an impairment of the homeostatic systems modulating metabolism. Indeed, these patients have normal plasma insulin levels and despite being overweight/obese, they also showed a normal HOMA index throughout the duration of the study.
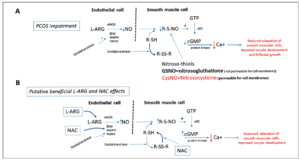
As for the mechanisms that might drive this important modification of the metabolic setting in hyperinsulinemic obese PCOS patients, it has to be stated that the four complementary substances we administered each have the ability, singly, to positively modulate insulin sensitivity, reducing IR in PCOS patients (12, 30-32). At present, considering the considerable impact of carnitines on the Krebs cycle in the modulation of beta oxidative processes (35), it can be inferred that administration of carnitines probably improved the low circulating carnitine plasma levels that PCOS patients have (34), and that this action permitted better cellular function at every level, from brain (i.e. metabolically sensitive hypothalamic areas) to the peripheral areas (i.e. fat, muscular and liver tissues) (35). It is probable that both L-ARG and NAC administration acted synergistically on this positive modulation of carnitines. Indeed, these two compounds have been clearly demonstrated to be deficient in PCOS patients (33) who also show a marked proinflammatory state (36). Both L-ARG and NAC donate thiol groups and reactivate oxidate gluthatione, also improving nitic oxide synthesis (36) (Fig. 5). This mechanism has been demonstrated to be at the basis of a protective action on endothelial cells as well as at ovarian level, and it has also been reported to drive the improvement of insulin sensitivity in PCOS patients (36-38), thus avoiding the triggering of IR and hypertensive predisposition (37-39). It cannot be excluded that the above protective effect facilitated the occurrence of pregnancies in the eight patients that were excluded a few weeks after starting our study.
Although carnitines (12, 28, 29), L-ARG and NAC (13, 30, 31) have been demonstrated to modulate and reduce IR at high dosages (12, 30-32, 38), our present data support the positive synergistic role exerted by the combination of low dosages of ALC, L-carnitine, L-ARG and NAC on the improvement of PCOS metabolic parameters.
Bearing in mind that a recent review stated that nonalcoholic fatty liver disease (NAFLD) is very frequent in PCOS patients, and that the combination of PCOS with obesity and IR is a dangerous cocktail that, over time, triggers not only NALFD but also the occurrence of type II diabetes (40, 41), as an additional feature, we also evaluated the HIE in our patients (23). This index reflects the dynamics of both insulin and C peptide peptides from the perspective of their secretion and metabolic clearance. It is well known that after proinsulin is packaged into vesicles in the Golgi apparatus (beta-granules) of pancreatic cells of Langherans islets (42), the C-peptide is removed, leaving the A-chain and B-chain bound together by disulfide bonds, thus constituting the insulin molecule. While C peptide reflects the β-cell secretory capacity, its hepatic extraction, unlike that of insulin, is negligible (43, 44); instead, insulin is mainly cleared by the liver (44). It is clear that although the production ratio between insulin and C peptide is theoretically 1, the ratio that can be computed between plasma insulin and C peptide concentrations reflects greatly the clearance kinetics of both peptides and represents a clear index between the amount of available insulin in the circulation and its release and activity from the pancreatic cells, the last represented by C peptide. This ratio cannot be equal to 1 both in physiological and physiopathological conditions. In fact, when using catheterization of the hepatic artery that enters the liver, the patterns of C-peptide and insulin were qualitatively similar (45). In case of the typical hyperinsulinemia of obese patients this pattern changed in the hepatic vein according to the liver extraction of insulin (45).
Our study demonstrated that the AUC HIE, computed on the OGTT of obese patients, was greatly modified by the complementary treatment. The AUC HIE of the whole group of PCOS patients showed a significant reduction after only 24 weeks of integrative treatment. When considering the PCOS patients divided by their insulin response to OGTT, the hyperinsulinemic PCOS subjects showed a significant reduction of HIE after just 12 and a further reduction after 24 weeks. No changes were observed in the normoinsulinemic group, whose AUC HIE did not change during the 24 weeks of treatment. Interestingly, these patients had a lower AUC HIE than the hyperinsulinemic subjects in baseline conditions, but a higher one after 24 weeks.
Previous data from our group (24) demonstrated that plasma transaminase levels in PCOS patients were reduced by administration of the hepatic protector alpha lipoic acid, thus supporting the presence of impaired liver function in PCOS patients. The present data disclosed that the hyperinsulinemic group showed reduced insulin extraction at hepatic level in baseline conditions and that the integrative treatment improved this parameter together with increased peripheral insulin sensitivity and a reduced insulin secretion.
This observation is in perfect agreement with Morin-Papunen et al. (46), who reported that peripheral hyperinsulinemia in obese PCOS patients is mainly due to the combination of two events: the IR at peripheral tissue levels (i.e. muscle and fat tissues) and the lowered hepatic insulin extraction (46). The latter is greatly influenced by hepatic blood flow, which might not be constant (45) and could have been improved, in our study, by the vasodilatory effects of L-ARG and NAC, which improve hepatic metabolic dynamics. Nevertheless, it cannot be excluded that the integrative treatment we administered acted on liver function through an improved expression/synthesis of the hepatic insulin degrading enzyme (IDE), which is responsible of at least 70-80% of the clearance of circulating insulin (38). The fact that no changes were observed in normoinsulinemic PCOS again clearly supports the hypothesis that not all obese PCOS patients are the same in terms of the dynamics of insulin secretion, sensitivity and degradation.
In conclusion, our study supports the efficiency of a combination of complementary substances, namely as carnitines, L-ARG and NAC, on metabolic impairments in obese PCOS patients. This study provides the evidence that the synergistic effects of these complementary substances can improve insulin sensitivity and as well as liver insulin extraction and metabolic impairments in overweight/obese PCOS patients.
Disclosure
The authors have no conflict of interest.