Introduction
Menstruation, a natural physiological phenomenon, includes proliferation and desquamation of the uterine endometrium on a monthly, repetitive basis [1]. Regular menstrual cycles offer a window into women’s overall reproductive health. A majority of women from all cultures and socioeconomic levels, however, experience regular recurrence of various symptoms during the days prior to menstruation, with these symptoms usually subsiding following menstruation. The symptomatology alters behavior and well-being, and affects relationships with family and friends, and at work. This enigmatic condition appearing in the late-luteal phase is commonly known as premenstrual syndrome (PMS) [2,3]. Symptoms and perceived discomfort levels of PMS vary from woman to woman and range from premenstrual molimina, considered within the normal range of physiological changes, to a debilitating premenstrual dysphoric disorder (PMDD), which leads to significant functional impairment, diminishes the affected woman’s quality of life, and requires treatment [2–4].
A number of population-based surveys on the epidemiological prevalence of premenstrual complications have been conducted worldwide. Although research designs and methods differ between surveys, the findings have been reasonably congruent. They have shown that nearly 90% of women of reproductive age experience at least one cyclical premenstrual symptom [3,5–9]. Using American College of Obstetrics and Gynecology criteria, approximately 20–40% of women were found to have PMS [3,8,10]. A further subset, comprising 2–8% of women, have severe, disabling premenstrual symptoms and fulfill the strict PMDD diagnostic criteria [3,8,10]. An assessment of published reports suggests that the prevalence of clinically significant premenstrual dysphoric symptoms is probably higher, with 13–18% of reproductive-age women found to have dysphoric symptoms that cause distress and impairment. These women may lack only one symptom to meet the criteria required for a PMDD diagnosis [10,11].
The Menstrual Distress Questionnaire (MDQ) is one of the earliest validated tools used to assess premenstrual symptoms [12]. Various assessment tools, including paper-based questionnaires, web-based instruments, and smartphone applications, have also been introduced [10,13,14]. Some of these instruments were developed to determine the presence or absence of PMS/PMDD, or to evaluate the severity of symptoms using a Likert-type or visual-analog scale in a retrospective fashion, while others measure daily symptoms prospectively.
Since the severity of PMS, along with other painful and/or emotional states, is largely described subjectively, assessment is greatly influenced by the individual’s perception, personality, tolerance, and individual measure of what constitutes ‘severe’ [10,15]. In a retrospective assessment, a woman’s memory further affects her evaluation of the premenstrual symptoms she believes she has experienced. To achieve a reliable diagnosis of PMS/PMDD, the cyclicity of the symptoms is most accurately determined by prospective daily documentation for at least two menstrual cycles, given the interpersonal variability in symptoms between menstrual cycles. We should note, however, that prospective recording is not always carried out in clinical practice or research settings (for various reasons, including, for example, the risk of creating a burden for patients or subjects that might result in limited adherence to the program); furthermore, it is unrealistic for large epidemiological studies [3,10]. While recognizing the pros and cons of both retrospective and prospective assessments, researchers have established a methodology including the use of psychometric tools to achieve the purpose or to clarify the hypothesis of their PMS/PMDD research projects. However, it remains unclear how and to what extent the severity, variety, and frequency of premenstrual symptoms differ between retrospective and prospective assessments.
The present authors conducted a series of investigations to explore the etiopathogenesis of PMS/PMDD [16–18] and to develop preventive health promotion programs to manage premenstrual distress among women in the early reproductive-age stage [19–21]. The authors’ 2019 retrospective epidemiological study showed the prevalence of premenstrual symptoms and its related factors among college students [5]. As a secondary and follow-up study, we conducted the present investigation by additionally using a prospective experimental setting and comparing premenstrual symptoms, which the college students had recalled in the retrospective assessment, with symptoms measured in the late-luteal phase in the same subject group. The study further investigated whether the premenstrual severity the women actually experienced influenced the difference between the two assessments. The authors, in addition, scrutinized common symptoms in the late-luteal phase among college students with different degrees of premenstrual symptoms.
Methods
The Institutional Review Board of Shitennoji University approved the study protocol, including the retrospective and prospective approaches. All study procedures complied with the ethical standards of the Helsinki Declaration of the World Medical Association. All subjects received an explanation of the nature and purpose of this study. Before receiving any data about the experiments, all subjects provided written informed consent to participate in the study.
Study design and participants
Two hundred college students (mean age: 19.8 ± 0.1 years), who had responded to a campus advertisement, participated in this PMS research project and completed the cross-sectional study with a retrospective questionnaire assessment. In 2019, the authors presented detailed information regarding the retrospective part of the PMS study in Biopsychosocial Medicine [5]. In short, the subjects first underwent a brief face-to-face interview and completed a standardized health questionnaire regarding medical history, medication, current health condition, regularity of menstrual cycles, and lifestyle. Each subject then filled out the self-report MDQ [12] to retrospectively evaluate the prevalence and severity of subjective symptoms and discomfort experienced premenstrually [5]. Briefly, the MDQ explores 46 symptoms in eight categories: pain, concentration, behavioral change, autonomic reactions, water retention, negative affect, arousal, and control. The subjects rated their experiences of all 46 symptoms listed in the MDQ on a six-point scale ranging from no experience of the symptom to experiencing its most severe level. The total scores could, therefore, range from a minimum of 46 points to a maximum 276 points.
None of the subjects had been clinically diagnosed with gynecological problems, such as amenorrhea, dysmenorrhea, and endometriosis. None of the women reported taking oral contraceptives to control their menstrual cycles. No subjects suffered from psychiatric disease, and none had been clinically diagnosed with diabetes mellitus, hypertension, hyperlipidemia, or other lifestyle-related diseases.
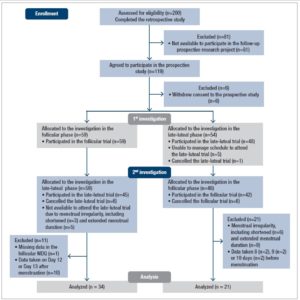
One hundred nineteen subjects agreed to further participate in the prospective part of the study after the retrospective assessment (Figure 1). In the prospective study, subjects were examined on two separate occasions: once during the follicular phase (the fifth to the eleventh day from the first day of menstruation) and once during the late-luteal phase (within seven days before the next menstruation), according to the authors’ previous studies [16–18,20]. On the basis of the subjects’ self-reported regular menstrual cycles, we determined the cycle phase during the experiments by the onset of menstruation, together with oral temperature (MC-172L, Omron, Kyoto, Japan) and concentrations of ovarian hormones, estrone, and pregnanediol-3-glucuronide (PdG), in a urine sample, taken early in the morning. Both estrone and PdG were indexed to creatinine (Cr) excretion [16–18,20]. Considering subjects’ menstrual cycles and availability to participate in this research project, the order of testing was randomized: 59 subjects were allocated to the investigation in the follicular phase followed by the late-luteal phase. The remainder of the subjects were tested in reverse order (Figure 1).
On the days of the follicular and late-luteal trials, subjects came to the laboratory between 8:00 and 12:00. Height and body weight of each subject were measured to calculate body mass index (BMI) as body weight divided by height squared. Subjects then filled out the MDQ to evaluate the prevalence and severity of follicular and late-luteal symptoms.
Statistical analysis
All descriptive and inferential statistical analyses were performed using a commercial software package (IBM SPSS Statistics Version 25; IBM Corp., Armonk, NY, USA). Internal consistency of the MDQ was evaluated by calculating Chronbach’s alpha coefficients. Paired t-test was performed to compare MDQ total scores in retrospective and prospective late-luteal trials. The effects of ‘group’ and ‘menstrual phase’, and their interaction, were evaluated using two-way analysis of variance (ANOVA) to investigate the influence of these two factors on clinical characteristics of subjects and on the MDQ sub-scores. Two-way ANOVA was also performed to explore the effects of ‘trial’ and ‘group’ on MDQ total scores. Unpaired t-test was utilized to compare the rate of change in MDQ total scores between retrospective and prospective late-luteal trials between two groups — Premenstrual Molimina Group and PMS Group. Pearson’s chi-square test and Fisher’s exact test were performed to compare the prevalence of premenstrual symptoms between the two groups. Values are reported as means ± standard deviations. Statistical tests were two-sided, and p < 0.05 was adopted as the level of significance.
Results
Figure 1 shows the flow of participants from recruitment through completion of the present study. Although 119 subjects agreed to participate in the prospective part of the study, six withdrew their consent and six did not attend the late-luteal trial in the first investigation. The authors excluded 52 subjects in the second stage of the prospective study for several reasons, including menstrual irregularity, unexpected changes in schedules, and missing data on the MDQ. This study, therefore, analyzed data taken from 55 participants (mean age: 20.2 ± 1.0 years) with regular menstrual cycles (29.3 ± 2.7 days), who completed two trials in the prospective study after the retrospective assessment.
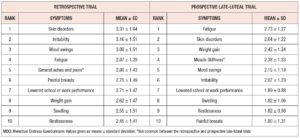
The values of the Cronbach’s alpha coefficient for the MDQ in the retrospective, prospective follicular, and prospective late-luteal trials were 0.95, 0.87, and 0.93, respectively. The MDQ total scores in the retrospective trial (88.3 ± 29.2) were significantly higher as compared to those in the prospective late-luteal trial (72.7 ± 19.3) [mean difference: 15.7, 95% confidence interval (CI) 9.1 to 22.3, p < 0.001]. The average value of the overestimation — the difference in the MDQ total scores between the two trials — was 23.7 ± 35.0%. Nine of the ten highest scoring symptoms were common between the two trials, regardless of severity. The exceptions were the items “general aches and pains” in the retrospective trial and “muscle stiffness” in the late-luteal trial (Table 1).
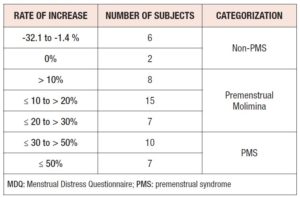
The MDQ total scores in the retrospective trial varied among subjects, ranging from 49 to 150. In other words, regardless of severity, the 55 subjects believed they experienced at least one symptom in the premenstrual phase. As Table 2 shows, however, two subjects showed no change in MDQ total scores between the follicular and late-luteal phases in the prospective study. In addition, in six subjects, MDQ total scores even decreased in the late-luteal phase compared with the follicular phase. Among the remaining subjects, the rate of increase in MDQ total scores from the follicular to the late-luteal phase ranged from 1.6 to 111.1%. The authors excluded the eight subjects mentioned above to scrutinize to what extent the severity of PMS (the increase of MDQ total scores from the follicular to the late-luteal phase) influenced overestimation of retrospective premenstrual symptoms, and also to explore which symptoms were more severe in the late-luteal phase. We then divided the 47 subjects into two groups — Premenstrual Molimina Group and PMS Group — in line with US National Institutes of Mental Health diagnostic guidelines together with our previous studies [3,17,18], and also including the quantification of premenstrual severity (a 30% increase in the intensity of prospectively measured symptoms from the follicular phase to the late-luteal phase) to define PMS. As additional reference data, the authors provide the following information. In a series of investigations, we have found a significant increase in sympathetic nerve activity and a decrease in parasympathetic nerve activity in the late-luteal phase when premenstrual symptomatology was substantially increased (>30%) as compared with the symptom-free follicular phase [16–18].
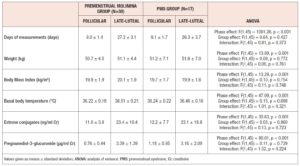
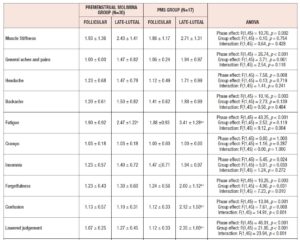
Table 3 shows the clinical characteristics of the subjects in the Premenstrual Molimina Group and the PMS Group in the follicular and the late-luteal phases. Body weight, BMI, basal body temperature, and concentration of estrone and PdG in urine were significantly elevated from the follicular to the late-luteal phase in both groups. Statistical analysis with ANOVA revealed no significant effect of group or interaction effect (group x menstrual phase) on these clinical characteristics.
Two-way ANOVA revealed that the MDQ total scores in the retrospective trial were significantly greater than those recorded in the prospective late-luteal trial both in the Premenstrual Molimina Group (retrospective: 79.2 ± 24.4, prospective late-luteal: 65.0 ± 13.4) and in the PMS Group (retrospective: 103.8 ± 28.4, prospective late-luteal: 92.1 ± 17.9) [phase effect: F(1,45) = 14.70, p < 0.001; group effect: F(1,45) = 22.33, p < 0.001; interaction: F(1,45) = 0.14, p = 0.710]. The authors also found no significant difference in the rate of change in MDQ total scores between the retrospective and the prospective late-luteal trials between the two groups [Premenstrual Molimina Group 22.6 ± 30.6 %, PMS Group: 14.2 ± 27.4 %, mean difference 8.4, 95% CI -9.6 to 26.4, p = 0.352]. The average value of the overestimation — the difference in the MDQ total scores between the two trials, among 47 subjects — was 19.5 ± 29.4 %.
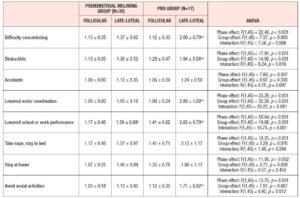
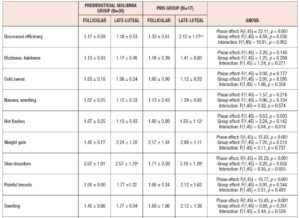
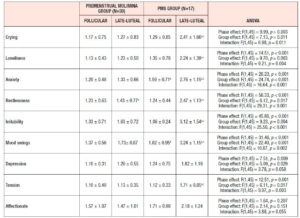
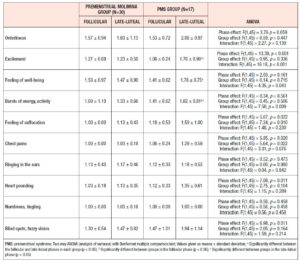
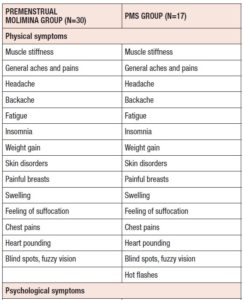
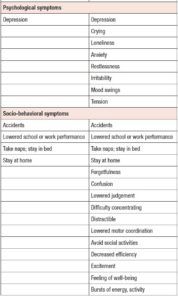
Table 4 shows the results of ANOVA investigation of the effects of menstrual phase and group and its interaction on 46 sub-scores of the MDQ in the prospective study. On the basis of these results, Table 5 lists symptoms which significantly increased from the follicular to the late-luteal phase. In the Premenstrual Molimina Group, the sub-scores of 19 symptoms (14 physical, 1 psychological, and 4 socio-behavioral) were significantly higher in the late-luteal phase as opposed to the follicular phase. The PMS group had more symptoms — 38 in total: 15 physical, 8 psychological, and 15 socio-behavioral — with significantly higher sub-scores in the late-luteal phase compared with the follicular phase.
We further scrutinized the differences in premenstrual symptoms evaluated in the prospective late-luteal trial between the Premenstrual Molimina Group and the PMS Group. As Table 6 demonstrates, the PMS Group had a greater prevalence of ‘moderate,’ ‘severe,’ or ‘extremely severe’ physical (fatigue) and psycho-socio-behavioral symptoms (forgetfulness, confusion, lowered judgement, lowered school or work performance, decreased efficiency, loneliness, anxiety, irritability, and mood swings) on the MDQ scale, compared with the Premenstrual Molimina Group. Statistical analysis further revealed that the PMS Group had the higher prevalence of ‘severe’ or ‘extremely severe’ emotional symptoms, including irritability and mood swings. Analysis additionally found no significant difference in the prevalence of the other 36 symptoms between the two groups.
Discussion
A number of epidemiological studies have used retrospective rather than prospective recordings to investigate the clinical features, prevalence, variety, and severity of symptoms of PMS — a cacophony of manifestations involving the mind and body [3,5–9,22,23]. A retrospective assessment tool is effective to make a preliminary diagnosis or conduct a large-scale field survey of PMS. Given memory bias, however, the accuracy level may be lower, unless retrospective recording is performed on day one of menstruation [10,24]. Taking these facts into consideration, the authors investigated how and to what extent premenstrual symptoms, which the college students had recalled in the retrospective assessment, differed from late-luteal symptoms that they reported prospectively. The present study found that nine of the top ten symptoms were common in both trials. However, the MDQ total scores, as an index of severity of the premenstrual symptom complex, was significantly greater in the retrospective trial than in the prospective late-luteal trial.
On the basis of the severity of the premenstrual symptoms experienced by the women, we further divided the subjects into two groups — Premenstrual Molimina Group and PMS Group — and found no difference in the degree of retrospective overestimation between the two. These findings indicate that women could, in general, recall their major premenstrual symptoms, but might overestimate (approximately 20% increase) the severity of these symptoms retrospectively.
In the prospective part of the study, the authors measured the scores of 46 symptoms on the MDQ in the follicular and late-luteal phases, following the methodology used in our previous menstrual-cycle studies [16–18,20]. We further calculated between-phase percentage change as an index of actual premenstrual severity, by subtracting the follicular from the late-luteal MDQ total scores, then dividing the value by the follicular score and multiplying by 100. Forty-seven of the 55 subjects, representing 85.5% of the sample, had at least one premenstrual symptom, regardless of severity. Based on the referential standard for PMS, a greater than 30% increase in the symptom scores from the follicular phase to the late luteal phase [3,10], we assigned 17 subjects to the PMS Group, and thus had a 30.9% prevalence rate of PMS. Despite the small size of this study, the prevalence rate of premenstrual symptoms we found was reasonably consistent with prior epidemiological research [3,5–10].
More than 200 symptoms relating to PMS have been reported over the last 50 years, falling in three main domains — physical, psychological, and socio-behavioral [2,3,8]. The top ten late-luteal symptoms among the participants in this study were: fatigue, skin disorders, weight gain, muscle stiffness, mood swings, irritability, lowered school or work performance, swelling, restlessness, and breast pain. Our results agree with those reported in earlier studies conducted in different countries, which indicate that regardless of ethnicity, women in their late teens and early twenties frequently experience such premenstrual complications [3,5–10,25–27].
As for the group comparison, the present study showed that the variety as well as the severity of symptoms were apparently greater in the PMS Group than in the Premenstrual Molimina Group. With the exception of “hot flashes,” the two groups experienced 14 common physical symptoms, irrespective of severity, as Table 5 indicates. In contrast, the PMS group had more severe psychological and socio-behavioral symptoms than the Premenstrual Molimina Group. The authors deem it plausible that, in addition to physical complaints, more serious psycho-socio-behavioral PMS symptoms could undermine overall mind and body health, and ultimately, diminish a woman’s quality of life. In fact, a series of investigations by the authors showed that women with PMS experienced autonomic imbalance — a significant late-luteal increase in sympathetic nerve activity and a decrease in parasympathetic nerve activity [16–18]. Moreover, our 2019 epidemiological study showed a negative association between the intensity of premenstrual symptomatology and subjective perceptions of health [5]. For women in the earlier reproductive-life stage, premenstrual symptoms can impair academic performance [6,22,23]. Furthermore, the symptomatology renders women more vulnerable to negative health outcomes in later years, such as postpartum depression [28]. The present study further suggests the need to develop an accurate, convenient, and, if possible, real-time PMS self-monitoring system. Health education programs dealing with the effects of ovarian hormones and menstrual cycles on biopsychosocial aspects of women’s lives and health could further assist in understanding this universal physiological phenomenon. Such programs could help college students to predict potential menstruation-related problems, to manage daily, academic, and/or social lives, and finally, to promote overall reproductive health [5,22].
The following limitations of the present study deserve mention. First, the prospective part of the study was conducted after completing the retrospective assessment. The severity of prospective late-luteal symptoms was significantly lower than that of retrospectively evaluated premenstrual symptoms. The authors cannot deny that the results of the prospective trial could be influenced by possible bias due to “learning” effects from the retrospective trial. Second, in the prospective part of the study, we examined subjects on two separate occasions: once during the follicular phase and once during the late-luteal phase. Since the severity of premenstrual symptoms can fluctuate even within the seven days before the next menstruation, the scores of the late-luteal symptoms we measured might not always reflect the most serious levels possible. Prospective recording of menstrual cycle-related symptoms is needed to detect frequently occurring symptoms premenstrually together with their severity. Finally, the present study included a small, selective, and unevenly distributed sample size. This could limit the generalizability of the study outcomes.
Conclusions
The findings of the present study indicate that women can recall their major premenstrual symptoms, but might overestimate (by approximately 20%) the severity of retrospectively as opposed to prospectively assessed symptoms. The prospective part of the study found that 85.5% of the subjects had at least one premenstrual symptom, regardless of severity. The prevalence rate of PMS was 30.9% in this study. Compared with the Premenstrual Molimina Group, the PMS Group had more serious psychological and socio-behavioral symptoms, in addition to physical complaints, which could undermine overall mind and body health. The present study thus further implies the need to develop an accurate and user-friendly PMS self-monitoring system, as well as educational programs on menstruation and its related problems, such as PMS, and ultimately, to improve the quality of life of women in the early reproductive-age stage.
Acknowledgments
The authors express their appreciation to all the volunteers for their dedicated participation in this study. The authors also thank Ms. Hitomi Nakata, a research assistant, for organizing and checking data obtained in the current study. This work was funded by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C) 18K11086.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.