Introduction
Hypoestrogenism and hyperinsulinism are independent risk factors for metabolic syndrome, dyslipidemia, endothelial dysfunction, inflammation, autonomic system disturbances, cardiovascular diseases, etc.[1].
Insulin resistance is defined as decreased sensitivity and/or responsiveness to the metabolic action of insulin, responsible for promoting glucose disposal. The etiology of insulin resistance can be: genetic (mutations in the insulin receptor gene, presence of anti-receptor antibodies, impaired beta cell secretory capacity); acquired (eating habits, physical activity, smoking); aging (decreased of beta cell functional capacity, increased of body fat, decreased uptake of glucose into muscles, decreased response of insulin to glucose). The metabolic and immune pathway have evolved to be closely linked. Hyperinsulinisms in the menopause induces weight gain, promoting autoimmune diseases by increasing production of T helper lymphocytes, interleukin-1b (IL-1b), interleukin-18 (IL-18), beta cells antibodies production etc.
Primary signaling pathways includes the insulin/IGF, TOR and sirtuin networks; alterations in signaling pathways which alter their mitochondrial function and metabolic activity via genome proteostasis[2]. Hyperinsulinism decreases the number of insulin receptors, insulin-like growth factor (IGF) receptors, insulin like growth factor binding globulin protein (IGFBP-1) and sex hormone binding globulin (SHBG). Insulin resistance elevates blood pressure [3].
Adipose tissue distribution changes to the central type in the menopause due to decreases in lipolysis inhibition, uptake of glucose into muscles and response of insulin to glucose, and to increased hepatic gluconeogenesis [4]. Visceral fat is reported to be associated with a high lipolysis rate, i.e., breakdown of triacylglycerol into glycerol and free fatty acid (FFA), which results in increased flux of FFA to the liver and enhances hepatic insulin resistance[5].
Different sleep-wake cycle patterns are genetic and sex dependent. A set of “clock genes” (PER1 and BMAL1), as well as estradiol receptors present in the suprachiasmatic nucleus in the hypothalamus, are influenced by photic information from photosensitive retinal ganglion cells and affect sleep habits in women [6]. Menopausal sleeping disorders exert unfavourable effects on insulin sensitivity.
The typical menopause symptomatology (sleeping disturbances, insomnia, anxiety, depression, lack of concentration, etc.) makes the female body more vulnerable to stressors, further increasing insulin resistance, levels of cortisol, cytokines, and oxidative stress and influencing telomerase length [7]. Oxidative stress causes damages to the cellular components such as proteins, lipids, and DNA, and increases tumor necrosis factor and IL-1b [8]. Estradiol therapy modulates the expression of antioxidant enzymes.
A US National Health and Nutrition Examination Survey showed that postmenopausal status increases risk of metabolic syndrome by 60% [9]. A study by Ren Y et al.[10] in 8191 menopausal women, 56 years of age, showed that risk factors (increased body mass index (BMI), increased waist circumference, increased triglycerides, hypertension) induced diabetes mellitus as a fourth cause of all mortality in women in the USA.
Menopause hormone replacement therapy, initiated in a timely fashion and individualized, can decrease or delay all unfavourable risk factors and improves quality of life.
This study was performed in order to establish out the route of estro-progestagen menopause hormone therapy (MHT) administration most effective on insulin secretion and sensitivity.
Subjects
Group I: 86 menopausal women, 51.3+2.1 years old age, amenorrheic period 2.1+2.1 years, BMI 25.2+2.5 kg/m2, waist circumference 83.5+9.9 cm, treated with 28 triphasic tablets (oral estradiol hemihydrate 2 mg for 12 days in the first phase; followed by 2 mg estradiol hemihydrate and norethisterone acetate 1 mg for 10 days in the second phase; estradiol hemihydrate 1 mg for 6 days in the third phase) (Trisequensâ tbl.)â;
Group II: 72 menopausal women, 49.8+2.3 years of age, amenorrheic period 2.5+2.5 years, BMI 24.1+2.9 kg/m2, waist circumference 82.5+9.2 cm, treated with transdermal patches in two phases: Estracomb patches /4 Estraderm TTS 50 patches, each containing 5 mg of estradiol/day, twice weekly and 4 patches of Estragest TTS each containing 10 mg estradiol/day and 30 mg norethisterone acetate, twice weekly/
Group III: 80 menopausal women, 52.4+2.4 years of age, amenorrheic period 2.4+2.0 years, BMI 24.9+2.4 kg/m2, waist circumference 83.0+9.0 cm, received 17 b estradiol valerate 4mg and prasterone enanthate 200 mg once monthly intramuscular (Gynodian depot amp.).
Methods
We performed a retrospective clinical interventional study of parallel groups in Clinic for endocrinology, diabetes and diseases of metabolism, Clinical center of Serbia. Inclusion criteria: menopausal women, amenorrheic period over 12 months, follicle-stimulating hormone (FSH) level >40 IU/L, estradiol level <50 pmol/L. Exclusion criteria: uterine bleeding of unknown origin, breast carcinoma, any kind of malignancy, endometrial thickness over 5 mm, liver and kidney insufficiency, thrombophlebitis, porphyria, MHT in the previous 12 months, BMI over 30 kg/m2, low motivation rate.
Blood samples were taken at 8 a.m. to measure levels of FSH, luteinizing hormone (LH), estradiol, thyroid-stimulating hormone (TSH), prolactin, sex hormone binding globulin (SHBG), progesterone, testosterone, and dehydroepiandrosterone sulphate (DHEAS). Oral glucose tolerance test (OGTT) was performed at 8 a.m. with 75 g of oral glucose ingestion after a 12-hours fasting period. Glucose and insulin were measured every 30 minutes for 2 hours (5 measurements). All the analyses were carried out before MHT, after 6 months of therapy, and after 2 years of therapy. The serum concentration of hormones was measured by radioimmunoassay using commercial kits (Sigma-Aldrich, St. Louis, MO, USA).
Data were analyzed using standard descriptive methods (minimum, maximum, mean, ± standard error of the mean (SEM), modal, and median values) and analytical statistical methods (Student’s t-test, Chi-squared test, Fisher test, Mann-Whitney test, Kruskal-Wallis test, and Friedman test). All data are presented as mean ± SEM. In order to identify the most sensitive method for detection of insulin sensitivity and secretion special indexes were calculated. The homeostatic model assessment-IR index (HOMA- IR) was calculated as [fasting plasma glucose (mmol/L) x fasting plasma insulin (mIU/L)/22.5] [11]. The area under the curve (AUC) [12-14], both for glucose and for insulin, was calculated as total AUC minus the AUC basal release. The Matsuda index was also calculated also. Single-factor and multi-factor analysis of variance was used for repeated measurements, whilst the Wilcoxon regression analysis approach was applied for categorical variables. To investigate the relationships between the tracked parameters Spearman’s and Pearson’s correlation coefficients were used. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc, Chicago, IL, USA).
The study was approved by the local ethics committee (No 2650/XII-9), and complied with the Helsinki Declaration on Human Rights.
Results
No weight gain was observed with any route of MHT administration over the period of 2 years.
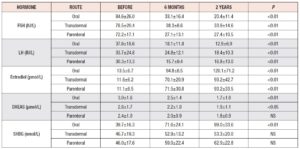
Significant hormonal changes are shown in Table 1. Estradiol continuously increased in all groups, while FSH and LH decreased, as expected. FSH was significantly lower in women on oral and parenteral MHT, as opposed to those treated using the transdermal route of administration. LH was significantly lower after 6 months of parenteral therapy compared with the other routes on the same time.
SHBG shown the highest levels after two years of oral MHT.
DHEAS continuously decreased on all routes of administration, but the least decrease was observed on parenteral route.
No significant changes were found for prolactin, progesterone, or TSH.
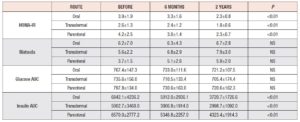
Table 2 shows the values recorded on insulin sensitivity tests. Significant decreases were found in the HOMA-IR index and insulin AUC with all routes of administration. Neither glucose AUC nor the Matsuda index showed significant changes. The insulin AUC correlated negatively with estradiol and SHBG. A positive correlation was observed between the Matsuda index and estradiol.
Discussion
Evidence has accumulated on the use of MHT in the primary prevention of coronary heart disease is accumulated. Estradiol therapy decreases adipocyte proliferation, and transcription of plasminogen activator inhibitor type 1 (PAI-1), C-reactive protein, interleukin -6, lipoprotein-a, and lipoprotein lipase transcription, increases hepatic excretion of apolipoprotein A and regulates PPAR gamma expression [15]. The metabolism of insulin plays an important role at the very beginning of the menopause because decreasing estradiol levels induces hyperinsulinism, enhancing proatherogenic and prothrombotic states.
The MHT doses administered menopause hormone therapy are very important in order to obtain the protective effect. The route of administration is important for bioavailability of estradiol and progesterone.
The oral route of administration is characterized by first pass transport through the liver, lower bioavailability, 4-5 times higher portal vein (compared with systemic) concentration with estradiol, higher levels of triglycerides (TG), high-density lipoproteins, and apolipoprotein A, and lower levels of low-density lipoproteins (LDL), and apolipoprotein B, and improved glucose level, stimulated renin and coagulation factors. It was reported in 24 meta-analysis that MHT significantly decreases lipoprotein a, the oral route of administration more than the transdermal one16.
The transdermal route excludes liver transport, and estradiol concentrations are lower. Also, TG, cholesterol, and LDL are lower and glucose metabolism is improved, whilst insulin resistance is decreased. No stimulation of renin was reported [17,18].
The parenteral route shows slow absorption from oil, maximal serum concentration of estradiol 3-5 days later, 37% bound in plasma to SHBG and 61% to albumin, estrogens in physiological relation and no changes in antithrombin III.
In the present study, estradiol continuously increased during the administration time. We founded significantly lower FSH levels with the oral and parenteral routes compared with the transdermal one, and the latter showed the lowest estradiol absorption rate.
Menopausal symptoms in all subjects disappeared. Prolactin, as a stress hormone, was not changed significantly. No changes in thyroid metabolism were found in this study, confirming that estradiol improve the immune response.
It is well known that lower SHBG levels increases metabolic risk. In this study oral therapy significantly increased SHBG levels, most significantly after 2 years. In agreement with results from others[19] , the levels were higher on oral compared with transdermal and parenteral therapy.
DHEA serves as a precursor for estrogens and androgens and acts in its own right through dedicated receptors. In the brain, DHEA is a neurosteroid that acts as a modulator of neurotransmitter receptors (gamma aminobutyric acid type A, N-methyl-D-aspartate and sigma 1 receptors) involved in neuroprotection, apoptosis, neurogenesis, catecholamine synthesis, anti-oxidation, and anti-inflammatory and anti-glucocorticoid effects. In human vessels binding of the DHEA to the cell membrane is associated with recruitment of Gα I2 and GαI3 that modulate intrasignalling cascades. DHEAS reaches maximum levels at the age of 30 years and decreases by about 60% at the time of menopause. This study confirmed a further decrease in DHEAS over a period of 2 years in women in the menopause. As regards the routes of administration, the parenteral route delayed DHEAS decrease, compared with the other two routes. Lower DHEAS has been linked to higher mortality from cardiovascular diseases and increased risk of ischemic stroke. Genazzani AR et al. adviced that DHEA replacement must be individualized in women who could benefit from this treatment [20].
The average weight gain, due to hyperinsulinism, in women at the beginning of the menopause is 3-5 kg. In this study, no significant differences in BMI after two years were found in any of the groups, indicating favourable effects of MHT. Kritz Silverstein D studying 671 women followed up for 15 years, also found no differences in BMI and waist circumference depending on route of MHT administration [21]. These findings confirmed that timely initiation of MHT protects against weight gain and all endocrine and metabolic processes in adipose tissue. We found that all routes of MHT administration improved the glucose AUC, in line with what was previously reported by Mauvais Jarvis F et al. [22].
Dong XC et al. [23] confirmed that estradiol improves glucose homeostasis through FOXO1 effects on gluconeogenesis, more than effects of re-uptake from muscles. Estradiol, influencing beta oxidation of fatty acid in mitochondria, resulted in recovered mRNA expression and increased activity of enzyme involved in fatty acid metabolism in an estrogen synthase knockout mouse [24]. Estradiol decreases production of hepatic glucose by activating the estrogen receptor signal pathway, independently of insulin receptors 1 and 2. It regulates eating behavior, increases lipolytic adrenal effects and decreases orexigenic peptides, protects against hepatic steatosis, and improves insulin sensitivity [25], as also confirmed in the present study. Bitolska I et al. [26] showed that estradiol hormone therapy increased insulin sensitivity, decreased proinflammatory signaling way and protected against beta cell apoptosis.
Our study found that the HOMA-IR index decreased with all routes of administration, most significantly with the parenteral route, confirming positive effects of MHT on insulin sensitivity. Brown M et al., too, found, that MHT significantly decreased insulin, compared with non-treated women [27]. Thurston RC et al. were reported that hot flashes, as the first marker of ongoing cardiovascular diseases, correlated with increasing HOMA index [28].
Considering the fact that HOMA is calculated only on basal glucose and insulin, tests describing insulin secretion during 2 hours would be more sensitive. The normal HOMA index is not sensitive enough to show hyperinsulinism. The area under the insulin curve is a more suitable test for showing this. A strength of the present study, and of the modern approach, is that it highlights the very important relationship between early detection of hyperinsulinism and favourable effects of MHT on insulin sensitivity. MHT administered by the oral route decreased AUC significantly, from 6842.1+4236.2 to 3720.7+1726.6; with MHT delivered by the parenteral route, it fell from 6570.0+4323.4 to 2777.2+ 1914.3. Transdermal administration of MHT also led to a significant AUC decrease, but less marked with the other two routes. This study suggested that MHT increases insulin sensitivity in early menopausal women. Li C et al. showed that oral 17 b estradiol and 0.5 mg norethisterone acetate, compared with placebo, significantly reduced glucose and insulin AUC [29], confirmed in this study with estradiol and 1 mg norethisterone acetate.
Hyperinsulinism requires treatment and lifestyle changes in order to prevent insulin resistance and metabolic syndrome, also earlier in life, i.e., in the reproductive period. In this study, women with normal BMI were tested. In overweight women with higher levels of insulin, we suggest adding metformin to MHT.
Our recent study of estradiol and drospirenone therapy in early menopausal women with grade I hypertension and hyperinsulinism showed significant decrease in insulin AUC and blood pressure, indicating that this may be a suitable treatment approach for early menopausal women [30] .
In their recent study, Mauvais Jarvis et al. showed that estradiol therapy improved islet oxygenation, revascularization and functional mass during pancreatic islet transplantation in a mouse model where estrogens were repurposed to improve pancreatic island transplantation [31]. The same authors confirmed that MHT prevents type 2 diabetes. The KEEPS study[32] showed that insulin resistance tended to decrease in MHT users.
Non-initiation of MHT at the onset of the menopause, or in the climacterium, can induce many diseases. The risks of the treatment have been grossly overstated and are hugely outweighted by many benefits. Recent studies have suggested a role for MHT in primary protection against coronary heart disease. Stevenson J et al. confirmed that MHT is clearly a very safe treatment, particularly in those women initiating therapy before the age of 60 years [33].
A crucial advantage of including OGTT in standard diagnostic work-up is linked to the fact that elevation of plasma insulin creates a prothrombotic state, inhibits fibrinolysis, irrespective of plasma glucose levels, and was found to produce a ninefold increase in tissue factor procoagulant activity, which was associated with an increase in monocyte tissue factor protein and mRNA, increasing plasma thrombin-antithrombin complexes, factor VII,
coagulant activity and platelet CD40 ligand [34]. Due to lack of knowledge of this cause, MHT is very often blamed for rare cases of thrombosis.
This study recommends OGTT, with insulin AUC calculation, as a part of standard testing in the routine practice, for selecting MHT dose and route of administration.
Estro-progestagen in MHT improves adaptive mechanisms and preserves blood vessels by means of anti-atherogenic, and anti-diabetogenic effects. MHT together with life style modifications can improve quality of life, protect against diseases and decrease mortality rate.
Disclosure statement
No potential conflict of interest was reported by the author