Introduction
Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity, predominantly, but not exclusively, in the pelvic compartment. It is an oestrogen-dependent chronic inflammatory condition that affects women of reproductive age, and it is associated with pelvic pain and infertility [1]. Endometriosis is one of the most controversial diseases in women; it seems to affect between 2% and 10% of those of reproductive age [2], but its true prevalence remains unknown [3]. Endometriosis is heterogeneous in nature, with lesions falling into three distinct phenotypes: (i) superficial peritoneal endometriosis, (ii) ovarian endometrioma (OMA), and (iii) deep infiltrating endometriosis (DIE). Additionally, adenomyosis (i.e., the presence of endometrial glands and stroma within the myometrium) is frequently associated with the disease.
Endometriosis is a disease known to be detrimental to fertility and the mechanisms involved are multifactorial [4]. Recourse to assisted reproductive technology (ART) is one of the therapeutic options offered to achieve a pregnancy; however, to date, published data on ART therapy outcomes in women affected by endometriosis are conflicting, and the factors determining pregnancy chances are unclear. The role of surgery in improving fertility outcome in women with endometriosis is a matter of debate; indeed, the risks of surgery and its potential damage to ovarian reserve must be weighed up against the complications associated with persistence of the endometriosis during ART therapy. Consequently, patients affected by endometriosis present a dilemma when choosing the appropriate therapy for infertility: first-line surgery or first-line ART? According to the European Society of Human Reproduction and Embryology (ESHRE) guideline reported by Dunselman et al. [3], surgical treatment of endometriomas before in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) is widely practiced, even though there is very little robust evidence to provide clinicians with adequate guidance. The ESHRE guideline underlines that in infertile women with endometrioma larger than 3 cm there is no evidence that cystectomy prior to treatment with ART improves pregnancy rates [5-7], but in women with endometrioma larger than 3 cm, they recommend considering cystectomy prior to ART, only to improve endometriosis-associated pain or the accessibility of follicles [2].
Patients with DIE may represent a separate subset. Although DIE has been blamed for lowering the pregnancy rates of IVF cycles, in this group of infertile women, the ESHRE guideline [3] offers no evidence to recommend surgical excision of deep nodular lesions prior to ART for the sole purpose of improving reproductive outcomes [8,9,10]. So, the challenge is to identify which women might benefit from an intervention, and which instead need another strategy in order to maximise their chances of pregnancy in the short term.
The aim of this study was to determine the impact of surgery on ART outcomes and clinical pregnancy rates (cPRs) in different endometriosis phenotypes and after IVF failure.
Materials and methods
General characteristics
Patients affected by endometriosis undergoing ART treatment at the Centre of Infertility and Assisted Reproduction of the Department of Clinical and Experimental Medicine of Pisa from January 2011 to December 2018 were retrospectively analysed. All patients underwent a complete clinical history and physical examination, biochemical analyses, and transvaginal ultrasonography (US). Age, height, weight and body mass index (BMI) were also recorded. Fertility investigations included hysterosalpingography, hysteroscopy, cycle day 3 measurements of serum levels of estradiol (E2), follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH), transvaginal US with antral follicle count (AFC), and semen analysis for the partner. Infertile patients with endometriosis plus other associated dysfunctions, such as polycystic ovary syndrome, hyperprolactinaemia, thyroid dysfunction, hypothalamic amenorrhoea, Cushing’s syndrome, and congenital adrenal hyperplasia, were excluded from the study; patients were also excluded if severe male factor infertility was present. In each patient, possible history of previous surgery was investigated. Previous surgery was defined as excision of superficial lesions, excision of deep lesions, bowel resection, or ovarian cystectomy. Infertile women were classified on the basis of endometriosis phenotype and subclassified on the basis of their history, i.e., as patients who had previously undergone ART or surgery followed by ART. All women had been proven infertile for at least 1 year. Diagnosis of OMA and DIE was based on physical examination, transvaginal US, and in some cases magnetic resonance imaging.
Study design
Between January 2011 and December 2018, 106 patients with endometriosis underwent 177 cycles of ART treatment at the Centre of Infertility and Assisted Reproduction of the Department of Clinical and Experimental Medicine of Pisa. We excluded 22 patients from this study because they had other associated female infertility factors, or because their male partner had severe oligoasthenospermia. We also excluded a further 6 patients who had previously undergone laparoscopy purely for diagnostic purposes or for superficial endometriosis without OMA, because considering the small number of patients and the lack of pathological US findings, we could not compare them with any category of patients. Finally, 78 patients were eligible for inclusion in this retrospective study.
These 78 patients underwent 131 ART cycles, with a mean of two cycles per patient (range 1-4): 37 patients underwent 1 ART cycle, 30 patients 2 cycles, 10 patients 3 cycles, and one woman underwent 4 cycles. Among these, 6 patients had surgery after two failed IVF cycles and subsequently underwent the ART therapy.
Patients who underwent first-line ART (n=22) were classified into two main phenotypes: patients with OMA and patients with DIE with or without associated OMA (DIE +/- OMA). Women who had undergone previous surgery (n=57) were also classified in two phenotypes: patients with a history of previous surgery for endometrioma only (OMA surgery) and patients with a history of previous surgery for DIE +/- OMA (DIE +/- OMA surgery).
According to the protocol of the Ethics Committee of Area Vasta Nord Ovest (Pisa, Italy), due to the observational, retrospective nature of this study, formal approval was not required.
ART procedures
Women were monitored and managed according to institutional clinical protocols. Various controlled ovarian stimulation (COS) protocols were used, with 150–450 IU/day of recombinant FSH or hMG (human menopausal gonadotropin) in a GnRH antagonist protocol, or a long agonist protocol. The gonadotropin starting dose and type of COS protocol were determined according to each patient's characteristics (age, BMI, AFC, and AMH level). Triggering of final oocyte maturation was carried out using 250 mg of recombinant hCG (Ovitrelle®, Merck Serono Europe Ltd, London, UK) when at least 1-2 follicles had reached a mean diameter of 17 mm. At approximately 36 h after triggering, transvaginal follicular aspiration was performed for oocyte retrieval. Volume, sperm count, forward motility and morphology were considered according to the World Health Organisation criteria [11]. Oocytes collected by vaginal US were incubated in oocyte culture medium (Sydney IVF Oocyte Wash Buffer; Cook Ireland Ltd., Limerick, Ireland). IVF or ICSI were performed as appropriate, depending on semen quality and the patient’s clinical history. Four to five hours after oocyte retrieval, in cases where IVF was performed, each oocyte was inseminated with 200 000-300 000 motile washed spermatozoa, while ICSI was performed as described elsewhere [12]. Fertilization was confirmed by the observation of two pronuclei 16–18 h after the fertilization technique. All the fertilized oocytes were transferred into a fresh cleavage medium (Sydney IVF Cleavage Medium; Cook Ireland Ltd.) and cultured until embryo transfer (ET), which was performed on day 2 or 5 under the guidance of abdominal US, using a K-Soft 500 Embryo Transfer Catheter® (Cook, Ireland Ltd.). From the day of the ET, all patients started luteal phase support with vaginal micronized progesterone, 200 mg three times a day (Prometrium®, Rottapharm S.p.A.), and intramuscular hydroxyprogesterone caproate every 72 h (Lentogest®, IBSA).
Statistical analysis
The categorical data were summarized by absolute, relative and percentage frequency; the numerical ones by mean and standard deviation. We compared the qualitative variables using the chi-square test and Fisher’s exact test. Comparison of the means was performed using ANOVA followed by multiple comparisons, or using Student's t-test. Significance was set at 0.05. All analyses were performed with SPSS version 25 technology.
Study population
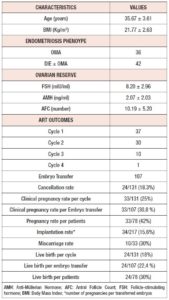
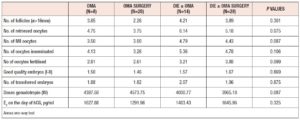
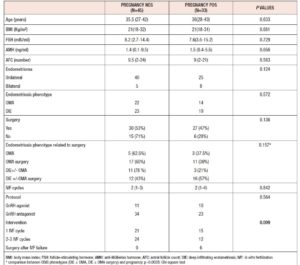
Between January 2011 and December 2018, 78 patients with endometriosis underwent ART cycles at our tertiary care centre. Demographic data and clinical characteristics of the study population are summarized in Table 1. The mean age of the general population was 35.67 years and they had a mean BMI of 21.77 kg/m2. Mean AMH level was 2.07 ng/mL, and mean AFC was 10.19. Table 2 shows the ovarian reserve markers in the different endometriosis phenotypes in relation to surgery (OMA; OMA surgery: DIE+/- OMA; DIE +/- OMA surgery). Patients did not differ in clinical characteristics or ovarian reserve parameters. However, patients who underwent first-line surgery showed a lower median AMH value compared with those who underwent first-line ART, irrespective of the type of surgery, although the difference was not statistically significant.
Results
ART outcomes
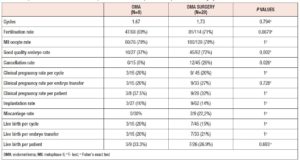
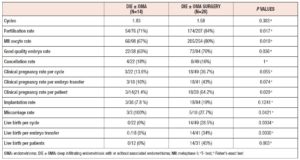
In total, 131 ART cycles associated with 107 embryo transfers were analysed. ART outcomes in the overall population are shown in Table 1 In total, 33 women (42%) became pregnant and 24 30 %) had a live birth. The cPR and the live birth rate (LBR) per ET were 30.8 % % and 22.4%, respectively. The cancellation rate was 18.3 % (n=24): 7 cycles (29%) were cancelled due to absence of oocytes at the ovarian pick-up, and 17 (71%) due to fertilisation failure. ART outcomes in the different endometriosis phenotypes related to surgery (OMA; OMA surgery: DIE+/- OMA; DIE +/- OMA surgery) are presented in Table 3 No statistically significant differences were observed between the four groups in the mean numbers of oocytes retrieved, metaphase II (MII) oocytes, fertilized oocytes, transferred embryos, good quality embryos, E2 values or total doses of gonadotrophins administered). However, patients with previous OMA surgery showed the lowest number of oocytes retrieved, despite receiving the highest dose of gonadotrophin compared with the other three groups. Table 4 shows the comparison of ART outcomes between patients with OMA and patients with previous OMA surgery. Patients who underwent first-line OMA surgery had a higher cancellation rate compared with those receiving first-line ART (p= 0.026). No difference was observed between the OMA phenotypes groups in fertilisation rate or oocyte metaphase II (MII) rate, while good the quality embryo rate was higher in the OMA surgery group compared with the OMA group. No significant differences were observed in pregnancy rate, live birth rate or miscarriage rate, per cycle, ET or patient, between OMA patients who underwent first-line surgery versus first-line ART. In Table 5 we compare ART outcomes between patients with DIE+/- OMA who underwent first-line ART and those with previous DIE+/- OMA surgery. No differences were found between the two groups in mean number of cycles or cancellation rate. On the other hand, significant differences were observed for fertilisation rate (71% vs 84%; p= 0.017), MII oocyte rate (67% vs 80%; p= 0.010), and good quality embryo rate (63% vs 76% p=0.036) between patients with DIE who underwent first-line IVF and those with previous surgery respectively. Patients who underwent first-line surgery for DIE had higher cPR and LBR per cycle, ET or patient, and a lower miscarriage rate compared with patients who underwent first-line ART- In particular, the DIE group and DIE surgery group had a cPR per cycle of 13.6% and 36.7%, respectively, and a PR per patient of 21.4% and 64.2% (p=0.020 ), respectively.
Prognostic factors of ART outcomes
The univariate analysis comparing patients who became pregnant and those who did not is presented in Table 6. Age, BMI, endometriosis phenotype, ovarian reserve parameters (FSH, AMH, AFC) and the stimulation protocol administered were not associated with a higher or lower chance of achieving pregnancy. Pregnancy appeared unrelated to surgery overall (p=0.136). However, patients without previous surgery seemed to have lower chances of pregnancy. This apparent advantage of surgery was clarified when patients were classified based on endometriosis phenotypes. Indeed, this advantage was only maintained for patients with a history of surgery for DIE. In addition, our data showed that undergoing surgery after IVF failure, rather than repeating IVF, offers a higher chance of pregnancy in endometriosis patients. Indeed, all patients who underwent surgery after IVF failure achieved pregnancy, and this finding was statistically significant (p=0.003).
Discussion
The aim of reproductive surgery in patients with endometriosis-related infertility is to improve the patient's ailment or quality of life. The management of endometriosis-related infertility depends on several factors: the presence of other causes of infertility, the patient’s age and general health status, the coexistence of painful symptoms, previous surgery for endometriosis, ovarian reserve, and duration of infertility. A combination of surgery with IVF has been suggested as a more effective approach in endometriosis-associated infertility [13-16], however there is uncertainty over the correct approach and timing to adopt for different patient phenotypes. In the past, when any ovarian lesion was observed on US before ART, the gold standard was to remove it, with the aim of “cleaning” the ovary and obtaining a better outcome, regardless of the patient’s symptoms. However, in the case of endometriotic cysts, laparoscopic ovarian cystectomy may lead to premature ovarian failure due to inherent dissection difficulties [16]. Fifteen years ago, Garcia Velasco et al., in a retrospective study, showed that removing endometriomas in women undergoing IVF did not improve cycle outcome; conversely, operated patients required higher doses of gonadotropins, showed lower production of E2 during ovarian stimulation, were exposed to the surgical risks of the laparoscopy, as well as to the costs, and showed a prolonged time to pregnancy [17]. On the other hand, OMA per se might be a cause of reduced ovarian reserve [18] and some authors suggest that surgery in an early stage on smaller cysts avoids progression of the disease, limiting the fibrosis and preserving the vascularization of the ovarian bed [19]. In our analysis, the patients did not significantly differ in terms of ovarian reserve. Since the patients who underwent surgery had a lower mean AMH level (not significant), in our series ovarian reserve did not predict a higher chance of pregnancy. This data partially conflicts with the findings of Maignien et al. [20] who observed that diminished ovarian reserve (AMH < 2 ng/mL, AFC < 10) was associated with lower pregnancy rates. On the other hand, it has previously been reported that an appropriate surgical technique does not determine a significant reduction of ovarian reserve [21]. In this regard, the surgical technique and the excessive use of bipolar coagulation could be the most important factors: an appropriate surgical technique, avoiding the use of bipolar coagulation of the ovarian border, does not jeopardise the ovarian reserve [22]. These conflicting data might be explained on the basis of the concept of “surgery and expertise”, clarified in a recent paper [19]. In this inspiring paper, in reference to the OMA “fertile battle”, it is explained that surgery should be performed in order to preserve ovarian reserve. Indeed, the level of expertise in endometriosis surgery inversely correlates with the amount of ovarian tissue inadvertently removed together with the endometrioma wall [21] and the experience of the surgeon may affect the live birth rate after IVF in women with surgically removed endometriomas [22]. One of the strengths of our study is that almost all the women included underwent surgery at our tertiary care centre. However, compared with the women with the OMA phenotype who underwent first-line IVF, in our study, the OMA surgery group showed a significantly higher cancellation rate, i.e., 26% (p=0.026), and in these patients the lowest number of oocytes was collected in spite of their receiving the highest doses of gonadotrophins compared with all the other groups. This result should not be underestimated. Despite the above findings, ART outcomes were comparable between the two groups, the only exception being the good quality embryo rate (higher in the surgically treated group) which suggests that the very presence of the endometrioma alters oocyte quality and therefore embryo quality. However, this did not affect pregnancy rate, suggesting that despite the lower number of oocytes collected in the OMA surgery group, the better oocyte quality might compensate. In conclusion, the best management of patients with ovarian endometriosis cannot be anything but a tailored treatment, and before making a strategic decision (surgery or ART), it is paramount to evaluate the ovarian reserve. In this regard, we agree with the view of Chapron who, in a recent paper, argues that the dogma that laparoscopic endometrioma cystectomy is the systematic first-line therapeutic option must be revisited [19].
On the other hand, the role of surgery in the management of infertile women with DIE remains a matter of intense debate. The available evidence is poor, as it derives mostly from case series, which may give biased conclusions. Even though DIE has been blamed for lowering the chances of pregnancy after IVF cycles, for infertile women with DIE, the ESHRE guideline [3] does not recommend surgical excision of deep nodular lesions prior to ART for the sole purpose of improving reproductive outcomes [8,9]. However, these women are often symptomatic, and require surgical treatment in any case.
In our study we observed that patients who underwent first-line surgery for DIE had a higher good quality embryo rate, cPR and LBR, and a lower miscarriage rate compared with patients who underwent first-line ART therapy, and these data were all statistically significant. However, due the small number of pregnancies in the DIE groups, these data must be treated with reasonable caution.
A prospective study [9] of 179 women compared IVF results in women with DIE-associated infertility submitted to extensive laparoscopic excision of endometriosis before IVF with those recorded in subjects not operated before IVF; it was found that the odds of achieving pregnancy were 2.45 times higher in the group submitted to surgical excision before IVF, even though those patients had fewer oocytes retrieved and required higher gonadotropin doses.
However, Bianchi et al. found that fewer oocytes were collected only in women who had associated ovarian endometriosis surgery, suggesting that deleterious effects are related to ovarian cystectomy rather than to DIE surgery itself [9] .
These data contrast with those of Mounsambote at al. [23], who, in a retrospective monocentric study, evaluated the impact on IVF outcomes of complete removal of endometriosis in cases of DIE without digestive involvement. They divided 72 infertile women with DIE without colorectal involvement who underwent IVF into two groups, on the basis of their history: “surgery” when they underwent complete endometriosis resection before IVF, and “without surgery” when they underwent IVF without endometriosis removal. They observed that ovarian reserve and cumulative pregnancy rates per patient were similar in both groups (40 % in the “surgery” group and 41% in the “without surgery” group; p = 1). Clinical pregnancy rates per cycle were also comparable (24% in the “surgery” group and 28% in the “without surgery” group; p = 0.67). The type of surgery performed was comparable between women who became pregnant and women who did not. However, age was lower in women who became pregnant and most pregnancies were obtained in women under 35 years old. In our study, the median age of the patients was slightly higher than that reported by Mounsambote et al. [23] and was homogeneous across all endometriosis phenotypes. In our group of patients, age, BMI and ovarian reserve were not prognostic factors for success, suggesting that the kind of intervention in endometriosis-related infertility patients who are comparable for clinical characteristics correlates well with the probability of success. However, we completely agree with the numerous retrospective and prospective studies [13,24,25] showing that female age is a major parameter influencing success rates in IVF-ICSI, with success rates clearly decreasing in women aged over 35 years. In their pregnancy-related nomogram, Ballester et al. [25] noted that in IVF-ICSI patient age and ovarian reserve were two independent prognostic criteria for pregnancy. So, in therapeutic decision making in infertile woman with endometriosis-related infertility, these two parameters might be carefully evaluated, and if surgery is deemed the best choice, fertility preservation should be considered. Interestingly, in our study, surgery after IVF failure offered the highest chance of pregnancy for infertile patients who had experienced that failure. Indeed, 100% of patients who underwent surgery after IVF failure went on to have a pregnancy. However, even though this value is significant, the low number of patients in our series partially limits its power, suggesting that these data need to be confirmed.
Similar results were observed by Littman et al. [26], who reported a 76% pregnancy rate after surgery in women who failed IVF treatments. However, that, too, was a small study, based on 29 patients, and most of the women who conceived had mild endometriosis (AFS 1–2) and underwent only one or two IVF cycles before surgery. Ballester et al. [14], in a retrospective study of 103 women with endometrioma (n=30) or endometrioma associated with DIE (n= 73), observed that when associated with endometrioma, DIE adversely affected cumulative pregnancy rates (82.5% vs 69.4%), and they recommended surgery when pregnancy was not achieved after three attempts at IVF. Centini et al. [27] also reported that the surgical removal of multiple lesions increased pregnancy and live birth rates, both spontaneously and after IVF. Interestingly, all patients who underwent surgery after IVF failure and had a pregnancy in a subsequent IVF cycle had DIE, and almost all had associated OMA. As a consequence, counselling should be integral part of the management, and it is imperative to underline the risks and benefits of the two interventions.
One of the limits of the present study is that a possible bias might be present between the patients who underwent surgery and those who not. Indeed, operated patients usually undergo more frequent medical checks and often the disease is more under control that in non-operated (and often less symptomatic) women, who frequently discover the disease in the course of concomitant infertility investigations. Additionally, operated woman are often on long-term medical therapy, such as progestin, after surgery and before ART in order to control the symptoms of the disease [28], avoid disease progression, and maximise IVF outcomes [29] .On the contrary, in non-operated women who discover the disease during fertility investigation, the disease may actually be more severe than it might appear [30,31].
Conclusions
As we wait for randomized well-designed clinical trials that might clarify the approach to the aforementioned “fertile battle”, the best management in infertile patients affected by endometriosis cannot be anything but a personalized treatment. In those with OMA, before making a strategic decision (surgery or ART), it is necessary to assess age, duration of infertility, ovarian reserve, and previous surgery. ART should be the first approach in the following situations: decreased ovarian reserve; associated infertility factors (male, tubal, etc.); OMA recurrence; or previous history of surgery for endometriosis. The indications for surgery are the rare situations of doubt concerning the nature of OMA on imaging workup, and the existence of intense pelvic pain associated with infertility, which is the main indication [19]. In DIE, surgery seems to offer the best chance of pregnancy in patients with endometriosis-related infertility, but to confirm our results, further randomized clinical trials are needed. In accordance with previously reported data [26], surgery seems to be a good option for patients who experience IVF failure. Finally, we suggest that when, on the basis of the concepts set out above, surgery is the mandatory choice in the management of infertile women with endometriosis, fertility preservation should be considered [32].
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: The authors declare no conflict of interest and have nothing to disclose
Key message:The therapeutic management of patients with endometriosis-related infertility should be tailored. First-line surgery in infertile patients with OMA does not improve the chances of pregnancy. In DIE, surgery seems to offer the best chance of pregnancy in infertile patients. After IVF failure, surgery should be considered.