Introduction
Premature ovarian insufficiency (POI) was formerly known as premature ovarian failure (POF), a term which referred to the cessation of ovarian function before the expected age of natural menopause (1). It has been questioned whether the old term, “ovarian failure”, is correct, since a significant percentage of affected women have evidence of follicular activity, may ovulate after the diagnosis has been established, and may even achieve pregnancy spontaneously (1). Today, therefore, it is preferred to use the concept of POI, which refers to this ovarian dysfunction as a variable, rather than a total and permanent absence of function (2).
POI is a relatively uncommon condition, which affects approximately 1% of women. Although it has a much lower prevalence in the population under 20 years, it is just as important and worthy of attention in this age group, given the implications of this diagnosis in a teenager — the serious health consequences and life impact in general. It is, indeed, a devastating diagnosis due to its extensive repercussions, especially in adolescents, but unfortunately there are still gaps in understanding of this disease entity and its management.
Definition
POI corresponds to hypergonadotropic hypogonadism of ovarian cause that occurs in women aged under 40 years (3). Other definitions are also accepted, such as: depletion or dysfunction of the ovarian follicles with cessation of menstruation (3), or the old definition of premature or early menopause, also in women under 40 years, to name just a few.
POI should not be confused with natural menopause, which occurs in women at around 50 years (50 ± 4) of age as part of their biological process; nor should it be confused with a decrease in ovarian reserve, which is a reason women seek consultation for infertility (4,5).
Epidemiology
According to other studies, POI affects 1 in 10,000 adolescents and young women under the age of 20 years, 1 in 1,000 under the age of 30 years, and 1-2 in 100 aged under 40 years (3). With regard to ethnicity, the SWAN study showed a prevalence of 0.1% in Japanese women, 0.5% in Chinese women, 1% in Caucasians, and a higher prevalence (1.4%) in African and Hispanic women (6).
Etiology
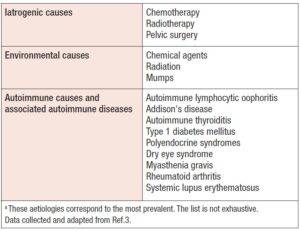
The etiology is diverse, as POI may be of genetic, iatrogenic, or autoimmune origin; however, in 60-70% of cases the cause is idiopathic (3). In adolescents, it is mainly iatrogenic, being linked to chemotherapy treatments and/or radiotherapy for malignant neoplasms, or due to some chromosomal alteration, as in Turner syndrome (TS) or fragile X premutation (4,7).
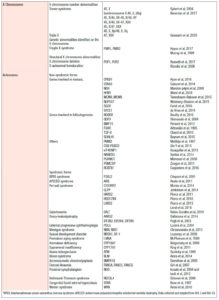
The genetic causes of POI are also diverse (3). They may involve the X chromosome — i.e., alterations in the X chromosome number, or structural or genetic abnormalities identified on the X chromosome — or they may be syndromic or isolated genetic causes, as detailed in Table 1. Non-genetic causes, on the other hand, can be autoimmune, iatrogenic, or environmental, among others (Table 2). It is important to bear in mind that 20% of patients, prior to the diagnosis of POI, have been diagnosed with an autoimmune disease (8).
The genetic etiologies include various chromosomal and other alterations, with more than 60 genes reported to be involved in POI. It is estimated that X chromosome abnormalities explain 12% of POI cases. TS is the most common etiology of X-linked POI, affecting 1 in 2,500 to 3,000 girls (3). It classically corresponds to an X monosomy (45, X0) that presents clinically with short stature, gonadal dysgenesis, and primary amenorrhea, among other manifestations (9). The mechanism that seems to explain the ovarian failure is an accelerated follicular atresia from the early stages of life. It should be noted that in cases of mosaicism, if the mutation percentage is less than 5%, it should not be considered a cause of POI (3). Excluding this syndrome, premutation of the FMR1 gene is the most frequent congenital cause of POI (9). Complete mutation of the FMR1 gene causes fragile X syndrome, which is the most common form of inherited intellectual disability. The premutation occurs in 1 out of 250 women, of whom 15-24% develop POI (9). There is evidence that women carriers of the fragile X chromosome premutation have a greater association of neurological, reproductive, endocrine, autoimmune, and psychiatric comorbidities (10).
Another important group of causes are iatrogenic, mostly cancer treatments. Both chemotherapy and radiotherapy can lead to a rapid loss of ovarian function, a condition known as “acute ovarian failure”, which, unlike POI, can be transitory (4). Chemotherapy produces a depletion of primordial follicles, altering the ovarian reserve and gonadal steroidogenesis (3). The gonadotoxic effects of chemotherapy will depend on its class, schedule, dose, duration, and the age of the patient (11). Radiotherapy will produce varying degrees of ovarian damage depending on the dose used, the irradiated field, and the age of the patient. Radiation therapy used to treat pelvic or abdominal neoplasms generally produces gonadal toxicity. In women younger than 40 years, doses of 20 Gy are sufficient to produce “acute ovarian failure”, whereas in older women this can occur with lower doses (12).
Pathophysiology
With regard to the pathophysiology of POI in adolescent women, to date two mechanisms have been identified: follicular dysfunction and follicular depletion. Follicular dysfunction may be due to inappropriate luteinization, autoimmune lymphocytic oophoritis, or, in rare cases, mutations in the follicle-stimulating hormone receptor. The term follicular depletion refers to the absence of primordial follicles in the ovary, although absence of remaining functional follicles cannot be proven (13). The mechanisms of follicular depletion are heterogeneous and can be modulated by genetic, iatrogenic, or autoimmune factors (14), as previously mentioned.
Diagnosis
The initial clinical presentation is an alteration of menstrual cyclicity, generally amenorrhea, which may be primary, with or without delayed pubertal development or secondary. In some cases, it may present as infrequent (oligomenorrhea) or frequent (polymenorrhea) abnormal uterine bleeding. It is estimated that POI is responsible for in 1-2% of cases of amenorrhea (4). It should be borne in mind that there are factors that tend to delay the diagnosis, especially in the first years after menarche, when menstrual disorders are more frequently observed and tend to be downplayed. However, even during adolescence, a 90-day amenorrhea is statistically abnormal, so evaluation is indicated (14). Other symptoms that may appear in association with hypoestrogenism are vaginal dryness, dyspareunia, hot flashes, and night sweats (13,14).
When faced with a patient with amenorrhea, pregnancy should be ruled out first of all (13). In cases of primary amenorrhea, an exhaustive anamnesis should be performed, looking for a family history of delayed puberty, infertility, and anosmia, the latter, in consideration of its association with Kallmann syndrome.
A personal history of chronic disease or treatments such as radiation therapy or chemotherapy should be obtained. The physical examination should record the degree of pubertal development, the patient’s nutritional status, and the appearance of the external genitals; it is also necessary to note any dysmorphic alteration that suggests a genetic or chromosomal condition, such as TS, in which we would find: short stature, low-set ears, small mandible, webbed neck, low hairline, short 4th and 5th metacarpal bones, and widely spaced nipples. In cases of secondary amenorrhea, it is important to focus on nutritional status, the characteristics of menstrual cycles, the possible presence of galactorrhea, and signs of hyperandrogenism, and to perform a complete gynecological examination (15). The analytical tests to be performed will vary depending on the type of amenorrhea, degree of pubertal development, as well as data obtained in the anamnesis and the findings of the physical examination. There are specific algorithms for each case, which will not be detailed in this article, but they basically involve measuring the following parameters: beta subunit of human chorionic gonadotropin, follicle-stimulating hormone (FSH), luteinizing hormone, 17β-estradiol (E2), thyroid-stimulating hormone (TSH), prolactin (PRL), and androgen levels and free androgen index, and performing a pelvic ultrasonography (15). This is important considering the differential diagnoses of amenorrhea.
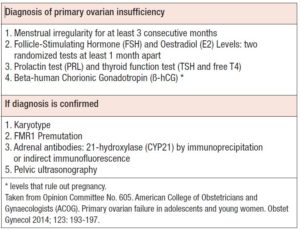
The condition is suspected in women under 40 years who have amenorrhea lasting longer than four months and levels of FSH in a range comparable to that found in natural menopause (two measurements at least 1 month apart): typically levels above 30-40 mUI/mL (4). In women under 20 years of age, there are no clearly established POI diagnostic criteria, which may delay a timely diagnosis, to the detriment of their health, especially with regard to the impact of the condition on bone and its structure (4,14). The American College of Obstetricians and Gynecologists (ACOG) propose an initial diagnostic and evaluation scheme for POI in adolescents that considers menstrual irregularity for at least 3 consecutive months, FSH and E2 levels, separated by two blood determinations at least one month apart; together with study of thyroid function (TSH, free T4) and measurement of PRL as part of the differential diagnosis of secondary amenorrhea (4,16). If the diagnosis is confirmed, a karyotype, FMR1 premutation test, and tests for adrenal antibodies (21-hydroxylase [CYP21]) by immunohistochemical techniques of immunoprecipitation or indirect immunofluorescence (4) should be requested as part of the study to establish an associated pathophysiological basis; together with a pelvic or, if feasible, transvaginal ultrasonography. The latter would allow observation of indirect signs of autoimmune lymphocytic oophoritis and some enzyme deficiencies, which occur with larger multifollicular ovaries (4,14). This information is summarized in Table 3. The role of anti-Müllerian hormone measurement in the context of POI is unclear (14). It is not necessary to request anti-ovarian antibodies as this test lacks specificity and diagnostic and prognostic value. In this sense, it is the determination of anti-adrenal or anti-CYP21 antibodies that allows an autoimmune origin of POI to be established (3).
Consequences
Women who experience an estrogen deficiency before the age of natural menopause have a higher risk of morbidities and early mortality. Estrogen replacement therapy appears to mitigate some, but not all, of the long-term health consequences of POI (17). Additionally, POI may be associated with several autoimmune diseases, or it may be the consequence of cancer treatments or prophylactic oophorectomy in women at high risk of developing cancer, which again can greatly affect mortality, and have significant consequences on life expectancy.
The complications associated with this pathology are diverse and present early. But it also has late consequences, which are the most relevant in terms of health. Some of them are detailed below.
Cardiovascular health
Cardiovascular mortality is known to be higher in women with early loss of endogenous estrogens. Cardiovascular events such as acute myocardial infarction or chronic pathologies such as coronary heart disease are more frequent in menopausal women compared with non-menopausal women, and a similar pattern is seen with ischemic strokes. The Framingham Study showed that cardiovascular risk (CVR) events tripled in the menopausal population compared with the non-menopausal population of the same age (18). Women undergoing bilateral oophorectomy before the age of 40 years have a high CVR; early estrogen replacement therapy can reverse this risk (17), in addition to having a protective role against ischemic strokes (19). This increased CVR is due in part to the loss of protection that endogenous estrogens confer against the formation of atheromatous plaques in the arterial wall; therefore, the younger the age at loss of ovarian function, the greater this risk is. Estrogen deficiency at a young age is added to the "lifetime" risk of cardiovascular disease (CVD). Also, serum cholesterol levels and obesity increase during natural menopause.
Bone health
The earlier POI occurs, the lower the individual’s bone density will be later in life (17). Young women with POI are known to have significantly lower bone mineral density (BMD) compared with women with regular menstrual cycles (20). A lower BMD is associated with a higher risk of osteoporosis and fragility fractures, which not only significantly alters quality of life, but is also associated with higher indirect mortality. It should be mentioned that performing bone densitometry in this age range is controversial, due to the poor correlation between the findings and the real risk of fracture (4).
Fertility
It is important to distinguish between low ovarian reserve and POI, as they are two separate entities with different management approaches. Low ovarian reserve is generally characterized by regular menstruation, but the presence of alterations in ovarian reserve tests; it can be caused by different pathologies that affect the ovaries, but in most cases, is a consequence of age (21). Low ovarian reserve is a condition in which the ovary loses its normal reproductive potential. Women with POI, on the other hand, face challenges much broader than fertility issues alone, and need appropriate management options (22).
As POI is characterized by cessation of ovarian function, loss of fertility is one of the key consequences of the diagnosis, and it is a major aspect of the impact of the diagnosis in adolescent women. An important part of the literature describes spontaneous pregnancies in women with POI and different specific etiologies. Restoration of ovarian activity can occur in women with POI, especially early in the natural history of the condition. This means that there is the possibility of spontaneous conception, which occurs in up to 5-10% of cases. In the event of a natural pregnancy, the cause of POI should be considered, due to its potential implications for the pregnancy and the unborn child (e.g., FMR1 premutation).
The central nervous system and neurological function
POI and other causes of premature estrogen deficiency have long-term negative effects on cognitive function (23). There are studies that associate an increased risk of dementia itself, and symptoms of parkinsonism, with oophorectomy performed before the age of menopause. In this last clinical condition, hormone replacement does not improve this association. However, the information regarding cognitive impairment is contradictory (24). Adolescents should, in any case, be advised to lead a healthy lifestyle in order to reduce the possible risks of cognitive deterioration (22). Notably, there are very few clinical studies evaluating the effects of POI on cognitive neurological function, including memory, dementia, and Parkinson's disease. Clinical evidence focuses on spontaneous POI, including genetic disorders and unknown causes (idiopathic), and iatrogenic POI, including oophorectomy and treatments for breast cancer. All this, together with the variety of etiological causes and the different pathophysiological phenomena responsible for ovarian insufficiency, makes it difficult to comprehensively assess the possible effects of this condition on the CNS and on neurological function (such as cognitive function and motor system deterioration), and this is particularly true in girls and very young women.
Mental health
Various psychological and psychiatric complications have been reported in patients with POI. These include personality disorders, insomnia, eating disorders, irritability, a tendency to isolation, and changes in mood. Additionally, POI is associated with an increased risk of developing depressive symptoms or major depression. Although these phenomena frequently accompany signs of altered ovarian function, they can also occur before its diagnosis, which suggests an overlapping pathophysiological association, and not a causal relationship (25). It is important that women, especially adolescents, with any of the aforementioned symptoms or disorders, consult or be referred to a specialist to establish adequate treatment.
Sexual and genito-urinary function
POIs can have direct or indirect effects on sexuality. The current literature cannot confidently answer many questions about female sexuality and other related topics such as arousal and orgasm in a way that is useful to affected women, particularly very young or adolescent women, some of whom may not yet have started their sexual life. Clinical research on the possible effects on sexuality in this patient population is scarce. Clinical research on the possible effects on sexuality in this patient population is scarce.
It is likely that significant sexual problems encountered in women with POI could be due primarily to physiological effects, or secondarily to the concomitant emotional burden of the diagnosis, especially in relation to its impact on fertility (26). It is highly unlikely that any finding is generalizable to women of all age groups and cultural and economic backgrounds, so data must always be analysed with caution, particularly in view of the paucity of interdisciplinary data in this regard. Women's ability to achieve sexual self-determination profoundly shapes their sexual outlook, both in relation to POI and in general. Furthermore, while most studies acknowledge that there are multiple factors at play in sexual experiences, from hormonal to spiritual, there is a lack of in-depth, quality clinical information in this regard.
Management and treatment
Currently available data on the management of POI in adolescent patients and young women are too scarce to allow evidence-based recommendations to be made, and to date, most recommendations have been formulated on the basis of expert opinion (27); even the special interest group on women with premature ovarian insufficiency, of the European Society of Human Reproduction and Embryology (ESHRE), does not address this topic directly (22).
Given that a diagnosis of POI can be confusing and an emotional challenge for affected girls and adolescents, especially given how it might affect their fertility and prospects of conceiving, it is important that both family members, especially the parents, and the doctor or health team in charge, address the situation carefully, referring to current recommendations (28).
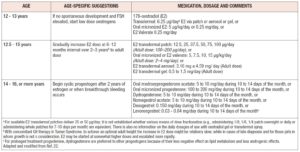
Once the diagnosis, implications, and future repercussions have been understood, it is essential, and a priority, to initiate replacement hormone therapy (HT), typically with estrogens and a progestogen, with the dual aim of correcting the clinical manifestations secondary to hypoestrogenism, including symptoms that deteriorate quality of life, and minimizing or avoiding long-term morbidities and pathologies at cardiovascular, neurological and bone levels (29). We here use the term HT to differentiate this treatment from menopausal hormonal therapy (MHT), as HT targets different age groups, with other comorbidities and management objectives compared with women in natural menopause. Traditionally, HT in young patients has included the use of exogenous estrogens, whose mode of use will depend on the degree of pubertal development or breast development reached, the aim of the treatment being to imitate the hormonal levels that would normally be present at the patient’s age and stage of development (4). In patients with POI who show incomplete or absent breast development, as well as primary amenorrhea, it is recommended to start HT with low doses of estrogens: approximately between 1/8th and 1/10th of the doses used in adults (14), and then gradually increase them until to reaching the average age of menarche or adequate weight and sexual development is achieved, which generally occurs within the first two years of starting the therapy. At that time, a progestogen must be added to allow adequate endometrial protection and associated cyclical bleeding. As regards the question of when to induce puberty, there is a consensus that this should take place at around 12 years of age, when most girls have started to develop sexual characteristics (22,27). In the case of patients starting full HT at the end of breast development, the treatment should include a dose of at least 100 micrograms per day of E2 or an equivalent, which can be administered orally, transdermally, or in some cases vaginally for formulations with conjugated equine estrogens in cream, or E2 can be administered through rings with proven systemic effect, always adding cyclically medroxyprogesterone acetate in a dose of 5mg to 10 mg orally for 10-14 days of the month (4,14), or another progestogen with a dose suitable to achieve a secretory transformation of the endometrium and to ensure adequate protection against hyperplasia or other proliferative disorders of the endometrium. With regard to estrogens, the transdermal route is preferable over oral administration as first-line management (20), and the use of E2 instead of ethinylestradiol (EE) or conjugated estrogens (30). If there is no contraindication, HT should be maintained at least until the natural age of the menopause (3,31) and can be continued beyond the age of 52 if there are reasons to consider extending its use (symptoms, bone density concerns or other, as scientific evidence proposes). The therapeutic regimen options for HT have been summarized in Table 4, adapting the proposal of the ESHRE group to include current available evidence and different expert opinions.
Combined oral contraceptives (COCs) with EE are not the first line of treatment because they contain doses many times higher than those used in HT (3), however, if they are used, the reasonable dose of EE for these patients is 30 micrograms in women under 25 years, as it allows better control of cyclical bleeding (and thus reduces abandonment due to poor control of this aspect), as well as minimizing a potential impact on bone mass gain in adolescents, although the evidence regarding this point is contradictory, with no deleterious effects found with very low doses of EE (20 mcg or less). Neither has the use of COCs been shown to be a risk factor for fractures (32,33); on the contrary, the study by Gazarra et al. concluded that continuous use of COCs, versus low-dose HT use, was associated with an increase in BMD in women with POI (34). Combined hormonal contraception (CHC) is especially useful in sexually active women, remembering that about 5-10% of women with POI may spontaneously become pregnant, a situation that should be considered in the care and management of the adolescent population (4,22,35). There are no studies evaluating the long-term effect of the use of COCs with E2 or other combined hormonal contraceptives such as patches, rings or injectables and their possible consequences on BMD and an eventual increased risk of fractures in the future.
There are various modifiable risk factors associated with the risk of fracture that are relevant to young women with POI, and should therefore be borne in mind. These include smoking, lack of exercise, calcium and vitamin D status, alcohol consumption, and low body weight (36). Although some of these have been associated with a low BMD, but not fracture risk, in women with POI (37), there is no reason to think that the association between these factors and the risk of fracture should not also apply to women with POI, therefore, it seems appropriate to advise on these modifiable risk factors. Furthermore, for the protection against eventual osteoporosis or osteopenia, adequate HT with estrogens together with a diet rich in calcium and vitamin D are sufficient, and in cases with inadequate calcium intake, a suboptimal vitamin D status, or decreased BMD, supplementation with both could be indicated (4,22,38).
There are no clinical trials on the use of bisphosphonates in women with POI, although there is evidence that these are beneficial in preventing chemotherapy-associated bone loss in women older than 40 years (39). Bisphosphonates remain incorporated in the bones for a long period of time, an aspect that must be borne in mind when considering their use in young women, and particularly in relation to future pregnancies. There is no direct evidence, but it is considered prudent to withdraw oral bisphosphonate treatment for at least 1 year in women with ovarian insufficiency who undergo egg donation to seek pregnancy. Risedronate has a shorter retention in bone than zoledronate. However, there are discrepancies in the recommendations regarding the use of this group of drugs, since use of these drugs for long periods presents adverse effects and uncertain safety profiles, and they should only be prescribed by an osteoporosis expert (4,22).
Another complex issue to address in these patients, especially in adolescents who have not had children, is fertility. Although spontaneous pregnancy can occur, there is no way of predicting in which women this might occur. That said, some favourable prognostic factors for fertility have been described, such as short periods of amenorrhea, autoimmune causes, and follicular ovarian activity observed on ultrasound (40,41). No intervention has been found to improve pregnancy rates in young women with POI (42), either through ovarian stimulation or ovulation induction, and the use of corticosteroids in autoimmune cases is ineffective, so these treatments are not recommended (43). In a subgroup of women, possibly with less advanced POI, estrogen treatment may increase the ovulation rate, especially in those patients with the presence of follicles on ultrasound imaging (44) and residual ovarian function (45).
Currently, fertility management in these women must be performed in highly complex reproductive medicine units. Only oocyte donation can achieve a high pregnancy rate, 25-40% per treatment cycle (22,43). Given that consecutive pregnancies after oocyte donation are associated with obstetric complications, their control and care by an appropriate obstetric team is recommended. There are no studies that show the effect of obstetric care on complications in these patients. While a sister (or other close relative) is often a suitable donor, sisters have a higher donation cycle cancellation rate. But if the cause of POI is genetic, the sibling also needs to be evaluated for POI before considering them as a donor. In women with TS there are special considerations regarding oocyte donation.
The literature contains publications dealing with in vitro maturation of ovarian follicles from women with POI. This is done using complex techniques with ovarian tissue fragments of tissue that are then transplanted by freezing to the pelvic cavity where the ovaries are normally found, achieving pregnancy with in vitro fertilization techniques (46,47,48). Other techniques, such as chemical treatment of ovarian tissue before grafting, have recently been explored, and in the future could provide options for fertility treatment in women with POI (49).
There are no validated tools for assessing the risk of developing CVD in women with POI, and the conventional ones are not suitable for women with this condition, as they have a higher relative risk of CVD compared with healthy women of the same age. However, investigation of CVR factors at the time of diagnosis may be indicated, since healthy lifestyle measures during perimenopause, such as smoking cessation, regular physical activity, and a healthy and balanced diet, improve health in later years, and should be advised (50). The ESHRE group of experts considers that blood pressure (BP), weight (with height), and cigarette consumption should be monitored at least annually, evaluating lipids, fasting glucose and HbA1c (22). A special mention should also be made of women with TS who have an excess of various CVR factors, including hypertension, obesity, glucose intolerance, and hyperlipaemia. Therefore, an annual screening for CVR factors should be performed. There are no clear recommendations on BP thresholds or targets for the treatment of hypertension in women with TS, but somewhat lower target values are believed to be desirable (22).
CVD and associated mortality could be prevented by the above measures in addition to HT. Different effects of HT are expected to exist in younger, healthier women (for example, women with early menopause who start treatment within 3 years of their last menstrual period) compared with older women (for example, women who entered menopause after 50 years of age, starting treatment 10 years after their last menstrual period), so the administration of estrogens would protect against atherosclerosis only if the vessels are healthy, without the negative effects on the cardiovascular system, especially prothrombotic phenomena associated with the destabilization of an established atherosclerotic plaque. (51). At this point the evidence favours the use of oral oestrogens in young women, although only one study showed a reduction in BP, better kidney function, and less activation of the renin-angiotensin system in women aged 19 to 39 years with POI (52).
To reduce cognitive decline and the risk of dementia in old age, young women with POI would benefit from timely estrogen treatment (53, 54, 55). There is a large body of basic scientific data, supported by biological plausibility, which suggests that estrogen use would protect an aging brain (56). Some limited observational data show an increased risk of cognitive decline after early (surgical) menopause that is reversed with treatment with estrogens. HT must be accompanied by lifestyle change to reduce the risk of vascular disorders associated with age-related cognitive decline and dementia in later life. Efforts should be made to reduce abdominal fat, hypertension, hyperlipaemia, and the risk of insulin resistance in middle age, therefore, it is advisable to stop smoking, exercise and eat a healthy diet (57).
Because POI affects multiple body systems, some health-related impact on quality of life is to be expected at some point. The effect can be from mild to severe, and transitory or prolonged, depending on a wide range of variables. Women who report a reduction in quality of life do not necessarily have to receive medical or psychological treatment. Some psychological distress in response to aspects of POI is normal. Coping with a level of adversity throughout life is intrinsic to human development. In some situations, a caring professional attitude may be the best form of clinical treatment. In young women, especially adolescents, the way in which the patient approaches her situation is very important, and it depends on both intrinsic and extrinsic factors, including physical health, current and past psychological health, age itself, parity, values and personal preferences, and access to social resources such as work, education, and supportive relationships. Management strategies should always include a complete evaluation of each individual case and most likely an interdisciplinary intervention. It is generally known that once HT adjustment is achieved, there is no evidence that additional medical interventions lead directly to significant psychological benefits. Psychological interventions associated with POI may produce positive benefits in quality of life and it is effective in improving self-esteem, reducing the levels of depression and anxiety in these patients, it does not improve the effects that the disease produces on the CNS (58).
In summary, HT is justified in women with POI and is particularly important in very young women in order to minimize serious impacts in terms of severe morbidity and early mortality related to prolonged estrogen deficiency, but at the same time, the treatment must be prescribed safely to avoid or minimize potential risks.
There are few studies comparing different HTs with estrogens in women with POI. The little evidence that does exist suggests that physiological sex steroid replacement regimens may be more beneficial than COCs and that the risks may be lower. The use of COCs in the female population in general poses risks that, although small, are well documented, so adequate counselling should always be carried out (59). However, if therapeutic compliance is improved through COC use alone, or if contraception is required, then this is a reasonable alternative. There are no data comparing the benefits or risks of E2 versus equine conjugated estrogens for women with POI, but the ESHRE expert group (22) considers the former to be preferable as it is more physiological. We found no studies comparing different types of progestogens for women with POI. There is evidence in postmenopausal women indicating that MHT with oral cyclic micronized natural progesterone was as good as cyclic or continuous medroxyprogesterone acetate for endometrial protection. Natural progesterone may have a more favourable cardiovascular profile and possibly a lower association with breast cancer risk (60).
Gonadotropin-releasing hormone (GnRH) agonists appear to be effective in protecting the ovaries during chemotherapy, in terms of maintenance and resumption of menstruation, treatment-related premature ovarian insufficiency, and ovulation, although well-designed randomized controlled trials with an adequate follow-up duration should be performed to clarify the effects of GnRH analogs in the prevention of chemotherapy-induced ovarian insufficiency, especially in different age groups or different regimens (61).
Also new is the use of stem cells, with their potential for self-renewal and regeneration, as a valid therapy for the treatment of ovarian insufficiency and, consequently, infertility. Recent studies have found that stem cell therapy can restore ovarian structure and function in POI animal models, providing an effective therapeutic method (62).
POI is a very heterogeneous condition. A number of genes have been found to be involved in altering processes such as gonadal development, DNA division and repair/meiosis, hormonal signalling and follicular development, immune function, and metabolism (63). However, they account for only a small proportion of patients and the majority remain without a genetic diagnosis. Identifying the genetic cause of POI can be of great benefit to affected patients and their families. Knowing a genetic cause can make it possible to screen family members, initiate HT earlier, minimize comorbidities, or even resort to egg cryopreservation to maximize future fertility potential. With a better understanding of the genetic cause of POI, a better indication of prognosis and fertility potential may be possible, allowing for better patient counselling and management. The discovery of new genes and pathways involved in the pathogenesis of POI allows us to understand the important processes necessary for ovarian function and can highlight the goals of new therapies and pharmacological treatments.
Conclusions
In adolescence, the diagnosis of POI is difficult and can be delayed by the mere assumption that irregular menstrual cycles are common at this age. However, this condition should always be ruled out in every girl or adolescent, especially when amenorrhea is present. It is also important to evaluate the multiple etiologies that we know today in the field of genetics, especially in POIs thought to be idiopathic.
Having an early diagnosis of POI facilitates the individualization of therapy, which is necessary since the health consequences of this condition differ from those of other causes of menstrual cycle irregularity. Although we have hormonal replacement therapies, the experience and use schemes are rather empirical, based on the hormonal therapies usually used in adulthood, which can be inefficient and cause unwanted and potentially dangerous side effects. Therefore, it remains urgent to optimize current HT regimens, and also to find new and effective prevention strategies for secondary causes of POI, especially in very young women. It is also necessary to continue to carry out new research that allows to show new genetic causes and thus develop new therapeutic frontiers for clinical practice, reducing the consequences that an eventual premature ovarian failure may have on the health of girls or adolescent.