Case report
A 39-year-old transmale person was referred by his mental health practitioner (MHP) for gender-affirmative hormonal treatment (GAHT) after the diagnosis of gender incongruence was made according the DSM-V criteria. On admission a healthy sportive and clinically unnoticeable person with a female phenotype was seen. Body mass index (BMI) was 27 kg/m2 and smoking habits was 10 cigarettes daily. Until referral no hormonal treatment was used. A subsequently available karyogramm showed 46,XX.
Gynecologically relevant information included: menarche at 12 years of age, nulliparous, amenorrhoea since one year with regular menstrual cycles beforehand, and only mild climacteric complaints. Regular gynaecological examinations in intervals of 2 years included PAP smears. The patient reported having been sexually active occasionally including penetrative intercourse from age 18 to 34. The sexual orientation was always female oriented.
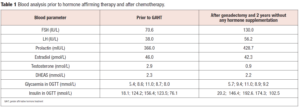
Apart from his mother, diagnosed at age 39 with premature ovarian insufficiency (POI), and suffering from gastric and esophageal carcinoma the family history was unremarkable. A native laboratory check from the intake visit revealed hypergonadotropic hypo-estrogenemia, glucose intolerance and insulin resistance (confirmed by a standard oral glucose tolerance test [OGTT] with 75 gr of glucose performed after 12 hours of fasting, in the morning at 8 am) (Table 1).
For study purposes, and outside routine procedures, ultrasound of several organs was performed including breast tissue, thyroid, upper abdomen and pelvic genital organs. This examination randomly revealed a fibroadenoma-like tumefaction in the lateral inferior quadrant of the left breast and a cyst in the upper lateral quadrant of the right breast. The diagnosis of transsexuality (ICD10: F64.0) was made and POI as relevant secondary diagnosis. After this routine medical checkup and the exclusion of contraindications for gender affirming hormone treatment, standard gender-affirmative therapy (GAT) with testosterone enanthate 250 mg biweekly IM was started.
Treatment trajectory
With the referral for gender affirming somatic treatment from a MHP and careful check-up according to the local Standard Operating Procedures (SOP), including features not all recommended by WPATH Standards-of-Care in power [1], GAHT was started with testosterone enanthate 250 mg IM in 2 week intervals. Satisfactory virilization slowly appeared according to the usual order. Eight months after starting GAHT and prior to the scheduled one-step gender affirming surgery (including bilateral mastectomy, thorax contouring, hysterectomy, bilateral adnexectomy, metoidioplasty, urethroplasty, scrotoplasty, and insertion of testicular protheses) mammography and ultrasound were performed. Radiologically the lesion previously described was again judged as a “Fibroadenoma” but size had doubled 9.2 x 4.3 mm. Also, the cyst was enlarged (7.2 x 5 mm). The mammography was again classified as BIRADS 3.
After bilateral mastectomy for gender-affirming purposes histology confirmed the preoperative imaging judgement: fibroadenoma of the left breast without signs of malignancy and a simple cyst of the right breast. The patient continued hormone treatment with testosterone enanthate 250 mg IM in prolonged intervals of 3 weeks. Endocrinological follow-up after the surgical procedures did not take place although scheduled.
Ten months later the patient reported pain in his left forearm and palpated enlarged axillar lymph nodes. Ultrasound revealed multiple hypoechogenic tumefactions in the subcutaneous tissue with diameters of 21.5 x 11.1 mm and 14.7 x 9.5 mm, and enhanced vascularization of the margins. Thoracic computed tomography (CT) revealed lymph nodes with a maximum diameter of 2.1 cm in the left axilla and multiple secondary pulmonary deposits bilaterally. No suspicious abdominal changes were found.
The patient underwent prompt surgery with tumor excision from the left lateral thoracic wall and axilla Histopathologically the specimen was classified as an invasive ductal adenocarcinoma. Subsequently molecular characteristics of the tumor became known, pointing to a poor prognosis: 1. Estrogen receptor (ER) negative: A II red score: PS-O, IS-O, TS-O; 2. Progesterone receptor (PgR) negative: Albert score: PS-O, IS-O, TS-O; 3. Androgen receptors (AR): 2% positive. AR subtypes were not studied; 4. c-erbB2 (Her2/Neu): 3+ (30%) and 5. Ki-67: gr 3 (50%).
Testosterone hormone therapy was discontinued by the oncologist. The patient underwent adjuvant chemotherapy with a standard FAC protocol (Fluorouracil, Adriamycin, Cytoxan). After 4 cycles another CT was done revealing no response on pulmonary metastases and chemotherapy was continued with a Taxol containing regimen. Not earlier than two years after his last endocrinological check (prior to his gender-affirming surgical procedures) and one year after finishing chemotherapy the patient was seen again by his endocrinologist. The patient showed all hormonal withdrawal effects possible, from extreme night sweats, hot flushes, mood swings to emotional irritability, anxiety, depression and insomnia. A weight gain of 9 kg was also registered. Hormone analysis showed: hypergonadotropic hypogonadism and worsened insulin resistance (Table 1). Also, gender dysphoria had reappeared.
Additionally a test for BRCA gene mutations was performed which initially appeared negative but with direct sequencing (Big Dye Termmator 3.1) a BRCA2 germ mutation on 10.exon 372.codon AAT CAT Asn His, heterozygous N 372H was detected. Also tests for MTHFR and PAI mutations were performed which also resulted positive. The patient received palliative treatment and psychological support and died only a few months later.
Discussion
This sad and unique case illustrates the importance of attentiveness for possible comorbidities and the necessity of being prepared for multidisciplinary care. In hindsight it must be noticed that breast cancer was diagnosed with a considerable diagnostic delay. From the first breast ultrasound to mammography 8 months passed and thereafter 12 months until the next mammography, instead of 6 months later according to BIRADS recommendations. However, there was no indication for performing invasive diagnostic procedures as repeated imaging did not show evidence of malignancy nor does the literature describe an elevated risk for breast cancer under GAT with testosterone. Gooren et al. [2] have reported in a cohort of 795 transmale persons receiving testosterone therapy only one breast cancer that corresponds to an increase of 5.9 per 100,000 person years compared to 154.7/100,000 person years in cis-women women and 1.1/100 000 person years in cis-men which is in the range of cisgender male persons [2].
During gender affirming thoracic surgery, as a principle, breast tissue is not completely removed. In the axillary region, and subcutaneously, small layers of residual breast tissue remain in situ on purpose to achieve better esthetic results of the thoracic contouring. Esthetic criteria and male appearance rather than oncologic criteria are the overarching goal of breast surgery in transmale persons.
To date, the lifetime risk for breast cancer in the female population is estimated from one to eight up to 12% whilst the risk in the male population is estimated only 0.1% [3]. Regarding the transgender and gender diverse population de Blok et al. [4] found 4 breast cancer cases out of 1,229 trans male persons and Brown et al. [5] 7 cases out of 5,135 indicating a very low incidence of breast cancer in transmale persons receiving testosterone therapy. Wiepjes et al. [6] from Amsterdam have shown with the support of the national cancer registry that transmale persons have a higher overall risk of breast cancer compared to Dutch cisgender men but lower compared to cisgender women. These data are in accordance with follow-up data from Serbia over a period of 32 years with only 1 person out of 211 transmales with breast cancer (Data not yet published).
Also, in the general discussion regarding the potency of steroid hormones to induce breast cancer, testosterone is under debate. In fact, so far, no clear association between testosterone and breast cancer has been found. A recent systematic review [7] found a total of 23 published cases worldwide of patients who developed breast cancer while on testosterone treatment. Four cases were cisgender men under testosterone supplementation and 18 cases were on testosterone for gender affirming purposes. Neither age nor duration of testosterone treatment showed any correlation. In our case, there were no signs for an elevated risk at the start of the transitional trajectory. Only at a later state it became apparent that there was a family history of hereditary cancer confirmed by the examination of breast cancer susceptibility genes. In light of this tragic case, one may argue that a thorough medical history, that includes family history, is always warranted.
Regarding the effects of androgens on breast cancer risk there are some possible biological pathways under debate. The first one is the aromatization of androgens to estradiol, thus leading to stimulation of breast cell proliferation and growth both in the peripheral fat tissue and in situ in the breast epithelium, or in the early occult breast tumor. Intratumoral aromatase serves as an intratumoral source of potent bioactive estrogen, irrespective of serum estrogen levels [8,9]. Androgen receptors are known to be expressed in 80-90% breast cancers [10]. In the absence of estrogen receptors (ER), androgen receptors can substitute them and initiate cell division in an ER-independent, but nuclear receptor-dependent manner [11]. A second possible pathway to be considered could be the conversion of testosterone by 5-alpha-reductase to dihydrotestosterone (DHT), thus directly affecting the androgen receptor in AR+ breast cancer. Our patient had 2% of androgen positive receptors in the breast tumor cells. In vitro studies have shown an inhibitory effect of androgens on breast cell proliferation and growth [12]. In vitro breast culture studies indicated that testosterone triggers both androgen receptor mediated antiproliferative and pro-apoptotic effects as well as inhibits ER-alfa activity and breast cancer cell growth [13].
Cancer cases positive for human epidermal growth factor receptor 2 (HER2) are rare in males, whereas 6-12% of the breast cancers in females express HER2/neu [14]. Our patient strongly expressed HER2/neu +++ which is regarded an unfavorable prognosis factor associated with increased invasiveness, increase tendency for metastasis and chemotherapy resistance.
Aromatase inhibitors have been widely used as the adjuvant therapy in postmenopausal women with ER+ primary breast cancer to block estrogens. Our patient had no estrogen and progesterone receptors positive cells suggesting that testosterone did neither induce breast cancer nor stimulate preexisting breast cancer cells. This conclusion seems to be in accordance with the literature available on breast cancer in transmale persons [15,16], and also with the 32 years follow up results of our Belgrade cohort [Data not yet published].
Micić et al. [17] have confirmed the anticancerogenic effects of metformin which activates AMPK,
stimulates the p53/p21 axis and inhibits fatty acid synthesis. Our patient did not receive therapy for insulin resistance. Although it is unlikely to have improved outcome, it may be regarded a wasted opportunity [18].
Male breast cancer represents less than 1% of all cancers in men [19]. A positive family history of breast cancer is associated with an increased risk of male breast cancer. Approximately 5-20% of male patients with breast cancer have a positive family history [20]. Female BRCA2 carriers have a lifetime risk for breast cancer of about 60%. This is about 8 times above the average. Male persons with mutations of the BRCA2 gene have 8% risk of acquiring breast cancer by the time they are 80 years of age. In the female population the homozygous genotype is reported to be associated with a 1.3 to 1.5-fold increased risk of breast and ovarian cancer [21]. In contrast, some other studies do not show any effect [22].
Polymorphism rs8176267 in BRCA 1 and N372H in BRCA2 have shown to be associated with breast cancer [23], as in our patient who also had a positive family history of cancer. Moreover, a meta-analysis [24] including 23 studies with 6,564 breast cancer cases and 7,891 case free controls explored the association between MTHFR gene polymorphism and breast cancer risk, suggesting also that folate deficiency may be related to development of breast cancer. The C677T polymorphism plays an important role in breast cancer, especially in Caucasians. Important to highlight is the fact that our patient had both mutations.
Our patient only received testosterone therapy for 1 year. With the occurrence of advanced breast cancer, hormone therapy was discontinued due to concerns about possible progression- triggering effects on breast cancer tissue; although current data appears not sufficient to establish a relevant correlation [8]. Unfortunately, this decision was made without consideration and involvement of the accompanying endocrinologists.
Our patient also suffered from POI with predominant hyperinsulinism. POI is characterized by oligo/amenorrhoea, elevated gonadotropins and hypoestrogenemia in women younger than 40 years of age [25,26]. A hypoestrogenic status induces hyperinsulinism, often accompanied by weight gain [27]. Hormone replacement therapy with estradiol and progesterone decreases insulin resistance [28], suppresses acute hypogonadal symptoms and prevents complications such as osteoporosis, vascular insufficiency among others.
In transmale persons under GAT with testosterone (with and without gonadectomy) considerable estrogen serum levels are noticed due to aromatase activity. Estrogens are regarded of importance for health maintenance of transmale persons and complete estrogen deprivation is not intended. In our case there was no reason to place greater emphasis on the patient’s POI at the beginning of GAHT.
GAT is still widely unknown among specialties not involved in transgender medicine, and - not uncommonly - dismissed as nonsense. As a consequence, cooperation with specialists in this evolving field frequently is not even considered with potentially devasting consequences. In our case the malignancy obviously showed aggressive features and the end of the patient’s life rapidly began to emerge. However, our patient unnecessarily suffered some years from the consequences of endocrinological negligence including re-occurrence of gender dysphoria.
Conclusion
There is no substantiated evidence to affirm that testosterone causes breast cancer. Adly et al. [29] showed that the effect of testosterone on breast cancer incidence disappears when the aromatized postmenopausal estrogen estrone is considered, supporting the hypothesis that aromatization of testosterone may be the primary contributor of tumorigenesis rather than the androgen per se [30,31]. It also appears worth to consider testing patients with breast carcinomas for insulin resistance. Although still under scrutiny, metformin may be considered a supplementary remedy for transgender males with breast carcinoma, as well as in cases of BRCA2 N372 H mutations, POI, insulin resistance, obesity or positive cancer family history as it may contribute avoiding the hypogonadal and hyperinsulinemic associated deleterious effects of carcinomas growth [18]. Multidisciplinary coordination and cooperation in multimorbid transgender patients may improve healthcare of this select group of patients and contribute to avoiding unnecessary physical and emotional suffering [32].