Introduction
Breast cancer (BC) represents the most frequent endocrine-related tumor with a global incidence of 24.5% and a mortality rate of 15.5%. An estimated 20-25% of new diagnoses occur in premenopausal women [1]. Progress in early diagnosis and in the development of new lines of therapy has improved disease-free survival but at the same time has led to an increase in treatment-related toxicities, with an inevitable deterioration in quality of life (QoL) [2]. In breast cancer survivors (BCS) with hormone dependent BC the proposed therapies, both in neoadjuvant and adjuvant settings, aim to decrease the amount of circulating estrogen hormones. Sudden estrogen deficiency, especially in premenopausal patients, is associated with severe menopausal symptoms. The most frequent side effects caused by anti-hormonal therapy are: hot flushes (HFs), genitourinary syndrome of menopause (GSM) [3] and osteoporosis. One of the consequences that especially characterizes young BCS is the worsening of the perception of their sexuality, also due to the fact that, although many patients perceive a worsening of their sexual health, they do not pose the problem to their oncologist or gynecologist or consider it normal [4].
It is well-known that the most effective therapy for vasomotor symptoms is systemic hormone replacement therapy (HRT). Systemic HRT is generally not advised for BCS, although hormone therapy use may be considered in women with severe HFs unresponsive to non-hormonal options, with shared decision-making in conjunction with their oncologists [5].
The aim of the present document is to review possible strategies to copy with treatment toxicities and adverse effects in young BCS, with special attention to the new perspectives.
Methods
This is a narrative review that focuses on the analysis of the new challenges in treatments for menopausal symptoms in BCS. We searched PubMed using the following keywords: breast cancer survivors, vasomotor symptoms, genitourinary syndrome of menopause, osteoporosis. Few results were obtained and therefore all were analyzed. Original papers selected for inclusion were independently reviewed by 3 authors (VEB, NA, MG). Only the publications written in English were considered.
Discussion
In Western countries, BC affects from 1 to 8 women who survive up to the age of 85. Four out of 5 new cases of BC are diagnosed in women over 50 years, with a peak in the age range of 50–64 years [1]. Young women present more aggressive cancer: higher proportion of late stage (Stage II, III and IV), high grade, node involvement, hormone-receptor (HR) negative and HER2 over expression [6].
According to the most recent guidelines published in the literature, standard adjuvant endocrine therapy (AET) should be continued for 5 years and it’s represented by tamoxifen in premenopausal and aromatase inhibitors (AI) in postmenopausal women. Patients with hormone dependent early BC remain at risk of relapse beyond 5 years. Estimation of risk relapse and prediction of treatment effect are important factors in selecting patients to be offered extended therapy up to 10 years, while the intermediate risk patients may benefit from extended therapy for up to 7-8 years.
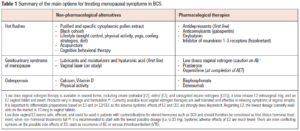
As reported in a recent paper [3] published by our group investigating the adherence to AET in BCS, side effects were reported by 81% of patients. Due to side effects, 21% of BCS considered discontinuing AET (72% in patients under AI and 28% in those taking tamoxifen). The addition of GnRH agonists to both tamoxifen and AI significantly increases the incidence of side effects. Patients who received adjuvant chemotherapy before starting AET reported a significantly higher incidence of side effects. Furthermore, premenopausal women were more likely to report side effects compared to older women [3]. The most common side effects among women taking tamoxifen are HFs and vaginal dryness, while arthralgia is often complained by women under AI [3,7]. Focusing on menopausal symptoms and QoL in BCS is thus an essential part of cancer treatment. The summary of the main options for treating menopausal symptoms in BCS is shown in Table 1.
Vasomotor symptoms
HFs are reported as one of the most prevalent and bothersome collateral effect observed in BCS under AET. According to previous studies about 50% of postmenopausal BCS report HFs during AET, 29% of which are considered as mild, 37% as moderate and 34% as severe, while among young BCS about 46% reported HFs with greater severity. BCS with HFs have significantly greater interference with daily activities and QoL, they report worsening of sleep quality, mood and concentration deficit.
Systemic HRT is considered the most effective treatment in the management of HFs but is not recommended in BCS [5]. Therefore, the new strategies are based on non-pharmacological treatments, with the use of herbal products, lifestyle changes, or pharmacological non-hormonal treatments, which do not impact on the risk of disease recurrence.
Non-pharmacological alternatives
Purified and Specific Cytoplasmic Pollen Extract
The purified and specific cytoplasmic pollen extract, is a combination of pollen, pistil extract (PI82) and vitamin E, characterized by a high antioxidant activity [10]. It has a similar effect of SSRI antidepressants, by inhibiting the reuptake of serotonin at the synaptic junctions. It does not contain phytoestrogens, has no estrogen-like effect on the endometrium and breasts and does not interfere with CYP450, being a safe option also for women under tamoxifen [11]. Its benefit on HFs has been demonstrated in several clinical trials, showing significant improvement in healthy women, with minimal side effects [10,12].
Preliminary data from a double blind, randomized versus placebo, prospective trial conducted in Italy showing good efficacy and tolerability in the treatment of HFs in BCS was presented at the International Society of Gynecological Endocrinology Congress (Florence, May 2022) [13]. Purified and specific cytoplasmic pollen extract seems to be a safe option for BCS [14]; although further in vivo studies on BCS are needed.
Black cohosh (Cimicifuga racemosa)
Black cohosh comes from a plant native to North America and has long been used to relieve climacteric symptoms. Results from the different studies are heterogeneous since the preparations with different parts of the plant or different kinds of extract can have different properties with respect of efficacy and also tolerability [15]. With certain formulations there has been the discussion of severe hepatic side effects which is controversial, but it is advisable to consider this discussion when liver function parameters during chemotherapy or AET are assessed.
It is effective in reducing HFs [16], with absence of estrogenic activity at the endometrial level and the lack of interference with circulating hormones [17,18]. The mechanism of action is not yet fully understood, but it is thought to have an anti-inflammatory, antioxidant, serotonergic agonist or selective estrogen receptor modulator (SERM) action, suggesting a chemopreventive effect for BC [15,19]. It seems to have a good efficacy and an excellent safety profile in BCS, not interfering with tamoxifen metabolism [15,20–23].
Lifestyle
Weight control in BCS after diagnosis seems to help containing HFs, while intentional weight loss after diagnosis does not seem to produce the same effect [24]. Studies have shown that physical activity can decrease the severity of HFs. In particular regular aerobic and resistance exercise training can lead to a decrease in subjectively experienced HFs [25], but avoiding it before sleeping is suggested.
In a meta-analysis conducted on more than 1,300 women, yoga resulted in an improvement of all menopausal, psychological and urogenital symptoms without adverse effects, even in BCS [26–28].
Furthermore, since flushing is triggered by high temperatures, albeit in the absence of scientific evidence, body cooling strategies have been proposed to reduce HFs: dressing in layers with light, cotton clothing and avoiding heat sources. Observational studies have shown that a diet rich in fruit, vegetables and dairy products helps reduce the frequency of HFs. On the contrary, alcohol, caffeine and hot spices should be avoided, especially in the evening [29].
Acupuncture
A large meta-analysis established the effectiveness of this technique in reducing the frequency and intensity of HFs, with an overall improvement in QoL and without significant side effects in healthy women [30,31]. Acupuncture has also demonstrated to be a safe and effective alternative for symptom management among BCS. These findings provide evidence-based recommendations for incorporating acupuncture into clinical BCS symptom management [32].
Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) is a form of psychological therapy based on the assumption that there is a close relationship between thoughts, emotions and behaviors. The psychotherapist and the patient both actively collaborate firstly by identifying thoughts, emotions and behaviors that come into play in situations of malaise and psychopathology; secondly, they actively collaborate to change maladaptive and dysfunctional habits of thought and behavior and to regulate their emotions more effectively. CBT is an effective technique in the treatment of HFs among both healthy and BCS. In the studies MENOS 1, conducted in BCS [33], and MENOS 2 [34], conducted in healthy postmenopausal women, CBT significantly reduced HFs. CBT is included among the recommended options to treat HFs in BCS in the most recent guidelines on menopause [35].
Pharmacological treatments
Antidepressants
The use of antidepressants belonging to the class of SSRIs and SNRIs are among the recommended treatment for HFs in both BCS and menopausal women [7,35]. In a Cochrane review published in 2010, SSRIs and SNRIs, together with clonidine and gabapentin, resulted in a mild to moderate reduction of vasomotor symptoms in BCS [36].
Among the SSRIs, paroxetine, sertraline, fluoxetine and escitalopram are the ones that have given the greatest positive results [37]. Only paroxetine 7.5 mg/day has been approved by the Food and Drug Administration (FDA) for the treatment of moderate-to-severe vasomotor symptoms also in people who cannot take hormones, such as BCS [38]. As for SNRIs, duloxetine and venlafaxine have demonstrated a benefit in reducing HFs [39]. All antidepressants should be prescribed at the lowest effective dose and possibly increase dose after at least two weeks.
The major reported side effects are nausea, asthenia, dry mouth, constipation and sexual dysfunction. These effects may lead to discontinuation of treatment in up to 50% of patients after 3 months [40,41]. SNRIs can cause an increase in blood pressure, and periodic monitoring of blood pressure is therefore advisable. Absolute contraindications to the use of this class of antidepressants include a pre-existing history of serotonin and neuroleptic syndromes or concomitant use of monoamine oxidase inhibitors. For BCS receiving tamoxifen therapy, it is important to remember that some drugs can interfere with its metabolism: among the SSRIs, paroxetine and fluoxetine should be avoided as they are potent inhibitors of the cytochrome CYP2D6, while citalopram and escitalopram have a mild inhibitory effect and can be used safely. Among SNRIs, venlafaxine and desvenlafaxine are the safest choices according to the minimal inhibition of the cytochrome CYP2D6 enzyme [42].
Anticonvulsants
This category of drugs, including gabapentin and pregabalin, are able to modulate the thermoregulatory activity of the hypothalamus, through modulation of calcium channels [43]. Gabapentin is an FDA approved antiepileptic drug that is commonly used to treat diabetic neuropathy and postherpetic neuralgia [35]. Results from clinical studies demonstrate a benefit up to 50% in reducing the frequency and severity of HFs [44–46] and, for gabapentin (900 mg/day posology), an improvement in the quality of sleep [47]. Compared with SSRI-SNRI antidepressants, gabapentin has more side effects, including drowsiness, shakiness, and dizziness. Pregabalin (50 - 300 mg/day) was equally effective, but the molecule has been less studied [48]. Gabapentin, but not pregabalin, is by menopausal guidelines among the recommended options for the treatment of HFs [35].
Oxybutynin
Oxybutynin is an anticholinergic drug approved by the FDA for treatment of overactive bladder symptoms. It acts on M1/M3 receptors in the brain, bladder and in small vessels, resulting in LH and FSH suppression and inhibition of vasodilation of small vessels. The effectiveness of oxybutynin has been evaluated in various studies. In a randomized-control study (RCT) published in 2016 [49] its efficacy was demonstrated in healthy patients, with a significant reduction in frequency and intensity of HFs compared to placebo at all weeks of treatment (p ≤ 0.007, all time points). At the end of treatment, 73% of women in the oxybutynin group and 26.1% in the placebo group rated improvement of symptoms (p≤ 0.001). Women treated with oxybutynin showed significant improvement in the global sleep index on the Pittsburgh Sleep Quality Index (p ≤ 0.023). The main side effect was dry mouth (52.1% oxybutynin and 5.3% placebo) with a drug discontinuation of 6.8%.
Another RCT published in 2020 [50] analyzed 150 women with and without BC with ≥ 28 HFs/week, lasting > 30 days, randomized to oral oxybutynin (2.5 mg twice a day or 5 mg twice a day) or placebo for 6 weeks. Patients on both oxybutynin doses reported greater reductions in the weekly HF score (p<0.005) and frequency (p<0.003) with improvement of overall QoL compared to placebo. Dry mouth was reported by 33% on oxybutynin 5 mg, 21% on oxybutynin 2.5 mg and 7% on placebo. Oxybutynin seems to improve vasomotor symptoms in BCS with a lack of interference of CYP2D6 enzyme being an interesting option for treating HFs in BCS [6]. It is by menopausal guidelines among the recommended treatments for HFs [35].
Clonidine
Clonidine is an antihypertensive drug, alpha-adrenergic agonist, little used, but capable of inhibiting HFs by reducing peripheral vascular reactivity. In a meta-analysis, clonidine was shown to significantly reduce the frequency of HFs even in patients who can-not take hormones [51]. The most significant side effects are represented by dry mouth, confusion, constipation, hypotension or hypertension in case of abrupt discontinuation of therapy.
NK1-3 neurokinin receptor antagonists
In menopause, the neuroendocrine signaling circuits are altered, resulting in dysregulation of the thermoregulatory center which causes the onset of HFs. Blockade of neurokinin B signaling with the use of an NK3R antagonist (NK3Ra) is proposed to normalize neurons expressing kisspeptin, neurokinin B and dynorphin (KNDy) activity and thus may help alleviate HFs in menopausal women [52].
NK3Ra trials show encouraging efficacy and tolerability/safety. NK3Ra were initially developed for psychiatric disorders with little success, then five molecules have been tested on HFs (pavinetant, SJX-653, MLE-301, fezolinetant, and elinzanetant). Recent work has focused primarily on fezolinetant, which is highly selective for the NK3R and has superior receptor occupancy at NK3R in the brain due to its high penetration and high free fraction in the cerebrum compared to earlier NK3Ra. Recent RCT data demonstrate that fezolinetant, taken as a once-daily oral tablet, can significantly reduce HF frequency and severity in diverse populations for up to 52 weeks. FDA recently approved fezolinetant as the first NK3Ra to treat moderate to severe HFs [35,53–56].
A recent multicenter double-blind controlled study evaluated in healthy women the efficacy of different doses of elinzanetant, another molecule similar to fezolinetant, with a significant reduction of HFs through 12 weeks of treatment versus placebo. It also led to clinically meaningful improvements in measure of sleep and QoL. The most frequently reported side effects were headache, somnolence, and diarrhea [57]. Another recently published RCT has analyzed the safety and pharmacokinetics of the same molecule on HFs in healthy women. A daily dose of 150 mg resulted in a rapid and marked improvement of HFs, without safety concern [58]. Studies on the efficacy of the molecule, also in BCS, are ongoing.
Genitourinary syndrome of menopause and sexuality
Sexual complaints are a common problem among BCS. They include: sexual desire disorder/decreased libido (23-64% of patients), arousal or lubrication concerns (20-48% of patients), orgasmic concerns (16-36% of patients) and dyspareunia (35-38% of patients) [7]. It is recommended that primary care clinicians should assess for signs and symptoms of sexual dysfunction or problems with sexual intimacy; should assess for reversible factors contributing to sexual dysfunction and treat when appropriate; should offer non-hormonal, water-based lubricants and moisturizers for vaginal dryness (first line treatment); should refer for psychoeducational support, group therapy, sexual counseling, marital counseling, or intensive psychotherapy when appropriate. In BCS, vaginal atrophy has been reported by 20% of premenopausal women and 42-70% of postmenopausal women, whereas dyspareunia has been reported by 10-16% and 27-39%, respectively [59]. Despite the prevalence of GSM, the condition is often inadequately addressed in medical practice [60].
Non-pharmacological alternatives
Lubricants and moisturizers
The first line of therapy to relieve the symptoms of GSM in BCS is represented by non-hormonal topical products, such as lubricants and vaginal creams. The creams form a bioadhesive layer on the vaginal walls and should be used regularly at least 2-3 times a week, regardless of sexual activity. The available data suggest that the efficacy of these therapies has not been sufficiently analyzed by studies comparing this category of products with estrogen-based vaginal therapies, however they have nevertheless underlined their usefulness compared to placebo in BCS [61].
A more recent study evaluated the non-inferiority of vaginal creams to topical E3 applied for 43 days, suggesting that in addition to improving vaginal dryness more effectively, it also improved dyspareunia and overall QoL, justifying its use in the first line for mild to moderate GSM and in BCS [62]. Lubricants, on the other hand, are mainly used at the time of sexual intercourse, and their regular use is associated in general with an improvement in sexual activity in both healthy women and in BCS [63].
Hyaluronic acid
Hyaluronic acid is a glycosaminoglycan naturally present in the body and one of the main components of connective tissue. In the amorphous matrix of connective tissue, hyaluronic acid helps maintain the degree of hydration, turgidity, plasticity and viscosity since it is arranged in an aggregate conformation thus storing a considerable number of water molecules. It is also capable of acting as an anti-shock molecule as well as an efficient lubricant, preventing damage to tissue cells from physical stress.
Hyaluronic acid is used in many dermatological products with the aim of healing wounds and promoting healing and skin re-epithelialization. Some studies have shown improvement in vaginal dryness and dyspareunia similar to placebo or local estrogen therapy [64,65] but to date, there is no evidence that hyaluronic acid-based products are more effective than products that do not contain it.
In view of its efficacy, safety (including patients with previous estrogen responsive cancer) and tolerability, the use of topical hyaluronic acid is recommended in the first line in patients experiencing vaginal dryness and dyspareunia [8,66,67].
Laser
Laser procedure is known to induce the production of new collagen and elastic fibers. It is a non-hormonal, easy-to-use, quick and safe procedure [68,69]. Two laser types are available for GSM: the micro ablative fractional carbon dioxide (CO2) laser and non-ablative erbium laser. Data in BCS on its efficacy on GSM symptoms and safety mainly derive from single-arm prospective studies, with only one RCT [70], with reassuring results in the short term both for CO2 and erbium lasers. However, further studies are mandatory to establish long-term efficacy and safety [71]. Even if many promising studies have been published on both types of laser in healthy women and in BCS [69,71–74], laser techniques are not yet considered as standard treatment for the management of GSM [71]. Further studies are mandatory to establish long-term efficacy and safety [71].
Pharmacological treatments
Systemic HRT is generally not advised in BCS, however low-dose vaginal estrogens or dehydroepiandrosterone (DHEA) can be considered in women with GSM after consulting with their oncologists if bothersome symptoms of GSM persist after the first line with non-hormonal therapies have been used [5].
Low-dose vaginal estrogen
Local estrogen treatment is more effective than systemic HRT in treating isolated symptoms of GSM [75]. Many oncologists do not consider vaginal estrogen therapy in BCS for the fear of increased cancer recurrence, the possible interference with AET and the fear of medical litigation [60]. Many types of estrogens with different pharmaceutical properties and doses are available; however, systemic absorption can occur with the conventional doses, particularly in case of atrophic vagina [59]. While E2 and estrone can be reversibly metabolized into each other, E3 is an end product of estrogen metabolism, with low affinity to the nuclear receptor and a short action. Promestriene also has a very low absorption after vaginal administration [76]. Local administration of E3 has excellent efficacy for vaginal dryness and even for urogenital symptoms and is recommended in some countries as first line, also taking into account the low risks of: VTE, stroke, myocardial infarction, BC, endometrial cancer or bleeding problems. All these risks are strongly reduced compared to E2 or CEE [9].
As for as CEE, there is concern in its use, some studies evaluated its association with a higher risk of ischemic stroke than E2 in postmenopausal Taiwanese women and its association with endometrial proliferation (higher risk of endometrial hyperplasia); despite this, CEE is still used in some countries, like USA and Italy, thanks to some formulations in combination with bazedoxifene acetate [77-78].
Low-dose local estrogen therapy is considered to have a low risk profile compared with standard doses because it produces very low serum levels when administered intravaginally [59]. Several studies in healthy postmenopausal women have demonstrated that low-doses of vaginal estrogens improve GSM in most treated women, with plasma E2 levels remaining in the range of postmenopausal women [79].
Ultra-low doses of vaginal estrogens are also available and have demonstrated to have good efficacy and negligible plasma levels, useful in postmenopausal healthy symptomatic women [80,81]. In BCS under AI, in whom circulating estrogens are completely deprived, even a little increase in systemic serum levels of estrogens might determine concern on AI efficacy. In vitro studies suggest that long-term E2 deprivation leads to an upregulation of estrogen receptor alpha and of growth factor pathways with consequent cancer cell hypersensitivity to low concentrations of estrogens [82].
A recent Danish observational cohort study showed how neither vaginal estrogen therapy nor systemic HRT was associated with increased risk of BC recurrence or mortality. A subgroup analysis revealed an increased risk of BC recurrence, but not mortality, in patients receiving vaginal estrogen (vaginal ring E2 2 mg; E3 1 mg; E2 10/25 mcg) during AI treatment [83]. In some countries a combination of E3 and lactobacilli is available, with excellent efficacy, and recommended also for BCS [84,85].
Prasterone (or DHEA)
Prasterone is biochemically and biologically identical to the endogenous human DHEA, a precursor steroid which is inactive and is converted into oestrogens and androgens. Efficacy of prasterone on moderate to severe dyspareunia and vaginal dryness was evaluated in a RCT (vaginal prasterone 6.5 mg/day vs placebo) in healthy women. As a result, dyspareunia decreased by 1.42 severity score unit from baseline or 0.36 unit over placebo (p=0.0002). On the other hand, moderate to severe vaginal dryness present in 84% of women improved at 12 weeks by 1.44 severity score unit compared to baseline, or 0.27 unit over placebo (p=0.004). Serum steroid levels remained well within the normal postmenopausal ranges. The only adverse effect reported was vaginal discharge [86]. Approval for the treatment of GSM was based on the results of the phase III ERC-231 and -238 trials. The study shows that the benefits of the therapy are still evident after 52 weeks of treatment. Prasterone was studied also in BCS. The RCT Alliance study [87] analyzed in 464 women (including also 285 BCS) the use of DHEA 3.35 mg compared to DHEA 6.5 mg and each compared to a plain moisturizer over 12 weeks. All arms reported improvements in either dryness or dyspareunia with no statistically significant differences between groups in any grade toxicity. Some women on DHEA may experience androgen related side effects, such as acne, unwanted hair loss and hair growth. Women on the 6.5 mg arm reported significantly better sexual health as evaluated by the Female Sexual Function Index (FSFI) (p<0.001).
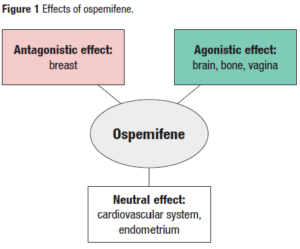
Ospemifene
Ospemifene is an oral SERM acting on the vaginal epithelium approved by the FDA in 2013 for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy (VVA) in postmenopausal women. The recommended dose is one tablet 60 mg once daily.
Ospemifene’s efficacy has been confirmed histologically [88] also improving in healthy women symptoms in the sexual sphere [89]. Several studies have demonstrated the safety of ospemifene, both through preclinical [90] and clinical data. In healthy women, no increase in spotting or abnormal bleeding has been shown, as well as a low risk of endometrial hyperplasia without atypia (0.3%) [89], no increased risk of VTE [91] and a protective effect on the bone [92,93].
Regarding the breast safety of ospemifene in healthy women, in different clinical studies, mammograms resulted normal after 52 weeks of treatment [94] and no cases of BC were found [95]. Regarding BCS, in the study of Cai et al. [96], patients with GSM and a history of BC in remission were considered; 58 of them were treated with ospemifene and 14,657 were not treated. 29% of the ospemifene treated patients and 75% of the controls had BC recurrence; there was no observed increase in risk of BC recurrence in patients treated with ospemifene.
Ospemifene is contraindicated in case of hypersensitivity to the active substance or to any of the excipients, history of VTE, unexplained vaginal bleeding, patients with suspected BC or patients undergoing active treatment (including AET) for BC, suspected or active sex-hormone dependent malignancy (i.e. endometrial cancer), patients with signs or symptoms of endometrial hyperplasia because safety in this patient group has not been studied. In conclusion, ospemifene is an effective and safe option to treat GSM in BCS at the completion of AET. However, further studies with longer follow-up are needed in this population.
Osteoporosis
The loss of bone mass is 7.7 times greater in women with menopause secondary to chemotherapy treatments or under AET than in those who go through menopause spontaneously [97–99]. Management of bone health in BCS consists in performing a baseline fracture risk assessment and evaluating bone mineral density (BMD) by Dual Energy X-ray Absorptiometry (DEXA) and dosage of vitamin D/serum calcium in order to prevent bone fractures.
Non-pharmacological alternatives
It is important to maintain an adequate daily calcium intake and vitamin D sufficiency. The oncologists should advise weight-bearing aerobic activities 3-5 days/week, such as tennis, stair climbing/descending, walking with intermittent jogging, activities that involve jumping; also, resistance training 2-3 days/week and flexibility training 5-7 days/week [100].
In the literature several studies show how exercise can also reduce fatigue and improve cardiorespiratory fitness, strength, and QoL in BCS. Resistance training and combined aerobic-resistance training interventions are recommended for the improving of general well-being. Exercise prescriptions should be delivered and initially supervised by trained exercise specialists [101].
Pharmacological treatments
To date, both bisphosphonates and denosumab are recognized as effective drugs in the primary prevention of osteoporotic fractures in BCS treated with AI. They have demonstrated their effectiveness in increasing BMD and in reducing the incidence of fractures, but they have also been shown to improve disease free survival (DFS) [102].
Bisphosphonate
Several trials have demonstrated that bisphosphonates prevented or reduced ongoing bone loss in women receiving AI [103]. In the ZO-Fast trial postmenopausal women receiving letrozole 2.5 mg daily were randomized to receive either immediate or delayed zoledronic acid. The group that received immediate zoledronic acid showed a statistically significant increase in BMD, while the delayed administration group demonstrated bone loss [104].
In 2015, the EBCTCG meta-analysis, evaluated the data of 18,766 patients and concluded that adjuvant bisphosphonate treatment can reduce the rate of bone metastasis from BC and improve BC survival (clear benefit was demonstrated only in postmenopausal women) [105]. The Cochrane’s latest systematic review, regarding the use of bisphosphonates in BC postmenopausal patients (under either AIs or tamoxifen), analyzed the results from 11 RCTs and has shown a reduced risk of bone metastases and a significant survival improvement in women treated with these agents versus placebo (HR 0.77, 95% CI 0.66 to 0.90; p = 0.001) [106].
Denosumab
Denosumab is a human monoclonal antibody directed against RANK-L which can determine a selective block of osteoclastic activity with a uniform efficacy on the whole skeleton, regardless of bone turnover, resulting in the inhibition of osteoclastic resorption of the cortical bone. Denosumab significantly increased BMD at 2 years in different skeletal sites compared to placebo (+7.6% lumbar spine, +4.7% total femur, +3.6% at the femoral neck, +5.9% at the trochanter level, +6.1% at the distal third of the radius and +4.2% overall; for all: p < 0.0001) [107]. Based on 268 primary endpoint events at database lock, denosumab significantly delayed time to first clinical fracture vs placebo (HR = 0.5 p < 0.0001). At month 36, an estimated 5% of patients in the denosumab group had experienced a fracture, vs 9.6% in the placebo group [108].
Updated results from an ABCSG-18 study published in 2019 and November 2022 showed an absolute 9-year DFS benefit of 3.5% points (79.4% vs. 75.9%) in the denosumab vs placebo group. Compared to bisphosphonates, denosumab has shown a better tolerability and a better adherence-to-treatment profile. No cases of mandibular osteonecrosis have been reported with the usual prescribed dosage of 60 mg subcutaneously every 6 months. To date, there is no definitive evidence regarding the duration of the treatment, although different guidelines agree in suggesting stopping denosumab at the end of the AI treatment. To avoid the rebound effect that emerges from some studies, the oncologist should prescribe a weekly oral bisphosphonate, such as alendronate, or a single infusion of zoledronate 5 mg for at least 1–2 years after denosumab suspension [102].
Conclusion
The adverse effects caused by anti-hormonal therapies, such as tamoxifen and AI, can become disabling and induce the patient to interrupt the therapy prematurely. For this reason, it is essential to investigate any symptoms and carry out accurate counseling with the patient for their most appropriate management. The most frequent toxicities manifested by BCS are HFs and GSM. Systemic HRT is generally not advised, however low-doses of vaginal estrogens and prasterone can be considered, with caution in AI patients. Many therapies have been shown to reduce the frequency and/or severity of HFs, such as the SSRI and SNRI antidepressants and gabapentin, making them a valid alternative for patients with contraindications to systemic HRT. Emerging data concern the new therapies based on neurokinin receptor inhibitors and oxybutynin, which appear to be valid alternatives for HFs, but studies are still ongoing. Ospemifene is an oral drug for the management of GSM that can be used by BCS after completion of AET. Vaginal laser is an emerging technique which is giving promising results, also in BCS. A further aspect to be considered in patients undergoing AET is the management of bone loss and the prevention of fractures, and in this field denosumab has demonstrated great efficacy in reducing the risk of fracture and in DFS.
The management of menopausal symptoms should be individualized according to the patient's preferences and should be directed towards those symptoms that affect women’s QoL the most. New strategies for therapy-induced toxicities management in BCS have been established and it is hoped that studies will be conducted to standardize their use and facilitate their management by clinicians.
Conflicts of interest
Nicoletta Biglia has received occasional grants from Sérélys® as a consultant. The other authors declare having no conflicts of interest.