Introduction
During the last decades, a growing body of evidence has pointed to the need of focusing on cardio-metabolic health with a sex and gender-oriented approach, in order to reduce the burden of cardiovascular diseases (CVD), which are the leading cause of mortality in both men and women worldwide [1]. The misconception that CVD affect female to a lesser extent than male population no longer exists, but knowledge gaps in pathophysiological mechanisms and clinical characteristics specific to women may hamper optimal management in the female population [2,3]. That being so, sex-specific (including events related to reproductive history) and gender-specific risk factors (including psychosocial modulators) should be evaluated in the cardiovascular assessment, alongside traditional risk factors, for a comprehensive approach [4]. When assessing the individual CVD risk, the full timeline of events related to reproductive milestones and/or disorders may help to predict women more at risk across the lifespan [5]. Starting from adolescence, both early (≤ 10 years) and late (≥15 years) menarche have been associated with increased CVD later in life [6]. During early adulthood, women with a diagnosis of polycystic ovary syndrome (PCOS) show an excess CVD risk compared to healthy peers [7] but this association becomes less clear approaching late reproductive and post-reproductive life [8]. Other gynaecological conditions, including endometriosis and uterine fibroids, have been reported to increase cardiometabolic risk factors and CVD; however, there is a need for further evaluation of the relative impact of these conditions which are known to represent low-grade inflammatory states, as well as of their treatments, including hormonal interventions (e.g. prolonged use of GnRH analogues) and pelvic surgery [9,10]. Concerning fertility, recurrent pregnancy loss may underlie a cardiovascular vulnerability, being associated with increased incidence of coronary heart disease [11]. Even a time-to-pregnancy > 12 months, defining infertility, has been related to a small increase in CVD after adjustment for traditional and sex specific risk factors [12]. When it comes to the association of pregnancy outcomes and cardiovascular health, evidences are far more robust. A recent meta-analysis, evaluating the impact of adverse pregnancy outcomes [APO] on CVD risk, found higher odds of experiencing a cardiovascular event in life following hypertensive disorders of pregnancy [HDP], preterm birth, gestational diabetes mellitus [GDM], placental abruption and stillbirth, with a consistent trend among women who had small-for-gestational-age/low birth weight infants [13]. However, an attempt to integrate a history of any APO, HPD or GDM in a prediction model of cardiovascular events has failed to improve risk prediction compared to traditional risk factors, highlighting the need for further research to determine how to use this valuable information to improve prevention in women [14].
At midlife, the menopausal transition represents a time of accelerated CVD risk, independent from chronological aging, being associated with atherogenic changes in lipid profile, decrease in insulin sensitivity, visceral adipose tissue deposition, endothelial dysfunction and increased inflammation [15]. Moreover, unfavourable cardiometabolic factors tend to cluster and metabolic syndrome becomes more prevalent in postmenopausal compared to premenopausal women irrespectively of aging [16], reaching a prevalence of 30% to 58% according to the studied population [17]. Menopause specific risk factors, including early onset of menopause (before 45 years), surgical menopause and severe vasomotor symptoms, have to be taken into account as CVD risk enhancers and managed appropriately [5].
That being so, menopause consultation represents a fundamental moment to evaluate in women cardiometabolic health and related risk factors, also taking into account their reproductive and obstetric history, in order to set the basis for a healthy aging [18]. Healthcare providers (HCPs) have the unique opportunity to gain valuable information regarding menstrual and pregnancy history of their midlife female patients, delineating a reproductive fil rouge which modulates individual cardiometabolic health across women’s lifespan [2]. The aim of the present retrospective observational study was to investigate metabolic phenotypes and their association with reproductive history in a clinical sample of midlife women, focusing on APO, with a special attention to HDP and GDM, and PCOS.
Methods
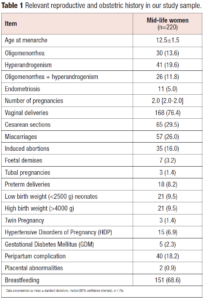
We assessed medical and reproductive history of 220 midlife women visiting the Menopause Clinic at our University Hospital (IRCCS Policlinico S. Matteo, Pavia, Italy) for the treatment of symptoms and prevention of conditions associated with menopause over the last 2 year. We included both perimenopausal and postmenopausal women, classifying them according to the Stages of Reproductive Aging Workshop (STRAW) criteria [19]. Data were collected retrospectively and a database was implemented starting from medical records. We considered only women who had signed an informed consent for data storage with research purpose. Incomplete cases for the selected variables were excluded. Only women who had at least one pregnancy followed by delivery were included in the analysis. We collected information about reproductive and obstetric characteristics evaluating number and mode of deliveries, history of HDP, GDM, miscarriages, foetal demise and the delivery of preterm, small-for-gestational-age and large-for-gestational age newborns. A probable diagnosis of PCOS was attributed to women with a long-term history of hyperandrogenism (signs and eventually documented high androgen levels) and irregular menstrual cycles with or without a self-reported polycystic ovary morphology (PCOM) during the reproductive years [20].
We defined the metabolic phenotype using the Adult Treatment Panel-III (ATP-III) metabolic syndrome definition [21,22] excluding the waist circumference ≥ 80 cm component due to its significant collinearity with body mass index (BMI). Women were classified as metabolically unhealthy if they had two or more of the following four ATP-III components:
(1) elevated triglycerides (≥150 mg/dL);
(2) low HDL-C (<50 mg/dL or use of medication for reduced HDL-C);
(3) elevated blood pressure (systolic/diastolic blood pressure ≥130/85 mmHg or use of antihypertensive medication);
(4) elevated fasting glucose (≥100 mg/dL or use of diabetes medication).
Women with less than two of the above four components were classified as metabolically healthy.
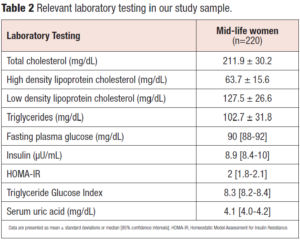
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the formula: HOMA-IR = [fasting glucose (nmol/L) * fasting insulin (µU/mL)/22.5] [23]. Values > 2.5 were considered positive for insulin resistance. The Triglyceride Glucose (TyG) index (cut-off of equal to 8) was calculated with established formulas according to previous studies [24,25]: TyG = Ln [Triglycerides (mg/mL) * fasting plasma glucose (mg/mL)/2]. Data were reported as number, percentage, frequency distribution, mean ± standard deviation (SD), and median (95% confidence intervals [CI]). Statistical analysis was performed by using parametric and non-parametric tests, as appropriate.
Results
General characteristics of the study population
Our study population of midlife women (n=220) displayed a median age of 54 years (95% CI: 53-55 years). Around one third (29.6%) of the sample (n=65 women) was in the menopausal transition (less than 1 year from last menstrual period) with 5.5% (n=12 women) in early menopausal transition (cycles > 7 days different from normal) and 24.1% (n=53) in late menopausal transition (cycles > 60 days and < 1 year). Established menopause was present in 70.4% (n=155) of women (n=83; 37.7% were in early postmenopause [final menstrual period, FMP 1-6 years] and n= 72; 32.7% were in late postmenopause [FMP > 6 years] and the median age at menopause was 50 years (95% CI: 50-51 years). In 94.1% of the cases (n=207) menopause was natural, with only 5.0% of women (n= 11) reporting surgical menopause and 0.9% (n=2) chemotherapy-induced menopause. The rate of menopause hormone therapy (MHT) use, including combined hormonal contraception (CHC) to overcome the transition, was 19.6% (40 women assuming MHT and only 3 assuming CHC).
The median body weight was 65.5 kg (95% CI: 64-67 kg) with a median BMI of 24.8 kg/m2 (95% CI: 24.2-25.4 kg/m2). A high rate of overweight (34.1%; n=75) and obesity (14.1%, n=31) was present together with a high rate of comorbidity: treated hypertension (22.7%; n=50); thyroid diseases (17.7%; n=39); autoimmune disorders, including thyroiditis (15.5%; n=34), established dyslipidemia (5.9%; n=13), diabetes mellitus type 2 (4.1%, n= 9) and osteoporosis (5.0%, n= 11). Some women were in regular follow-up for colon cancer (0.45%, n= 1) and breast cancer (0.9%, n= 2), polycystic kidney disease (0.45%, n= 1), antiphospholipid syndrome (0.45%, n= 1), stroke (0.9%, n= 2) and deep venous thrombosis (1.36 %, n= 3). Smoking habit was present in 20.9% (n=46) of the study sample.
Reproductive characteristics of the study population
Table 1 reports relevant reproductive characteristics in our sample of midlife women reflecting the reality of an Italian population referring to a tertiary centre for menopause care. Interestingly, we found a history of complicated pregnancy due to HDP and/or GDM in 9.1% (n=20) and we could suspect a positive history of PCOS in 11.8% (n=26) of our study sample.
Metabolic characteristics of the study population
Relevant laboratory testing to assess the metabolic phenotype are reported in Table 2. We stratified our population according to BMI, HOMA-IR, TyG index and cardiometabolic phenotype (metabolically healthy or unhealthy). A metabolically healthy phenotype was evident in 77.7% (n=171) of the cases; a TyG index > 8 was detected in 77.7% (n= 171) and a HOMA-IR > 2.5 in 31.8% (n=70).
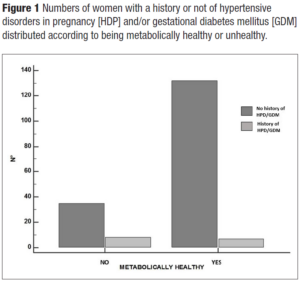
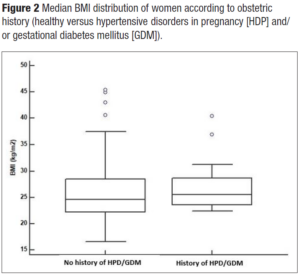
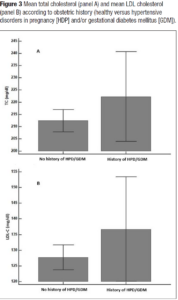
Hypertension was present in 22.7% of women (n=50). Interestingly, women with a positive history of HDP and/or GDM showed hypertension at menopause in 30% of the cases versus 22% of women with a negative history. Overweight and obese women were more represented (55%) in women with a positive history of HDP and/or GDM in comparison to those without (47.5%). Even HOMA-IR > 2.5 was more represented in women with a history of APO versus those without (45% versus 30.5%). Interestingly enough, even though the study sample of midlife women with a positive history for HDP and/or GDM was quite small, the metabolically unhealthy phenotype presented more cases of HDP and/or GDM (χ2= 6.5; p = 0.01) (Figure 1). Indeed, they had a slight tendency to have higher BMI (Figure 2). Even total cholesterol was significantly higher (p <0.036) (Figure 3, panel A), as well as LDL-C (p <0.02) (Figure 3, panel B). No association was evident between TyG index and APO. Even serum uric acid (SUA) displayed no differences.
We found a higher percentage of hypertensive women when a previous PCOS was suspected (26.9%) compared to women without probable PCOS (22.2%). Overweight and obese women were more represented (61.6%) in women with a probable history of PCOS in comparison with those without (46.4%). HOMA-IR > 2.5 was only slightly different between women with and without a probable history of PCOS (34.6% vs 31.4%). We observed a non-significant trend to display a metabolically unhealthy phenotype more frequently in midlife women with a probable history of PCOS (30.8%) with respect to those who did not (21.1%). There was also a non-significant trend to have higher BMI and triglycerides in women with probable history of PCOS. TyG index and SUA were superimposable in women with and without a probable history of PCOS.
Discussion
Findings from this clinical cohort of midlife women indicate that a history of pregnancy complications, specifically HDP and/or GDM, was more likely associated with an unfavourable metabolic profile, compared to women who did not report APO in their life, based on the evaluation of blood pressure, lipid profile and fasting blood glucose. When there is an alteration of these parameters, primary CVD prevention should be established [26]. In our sample, well-known cardiovascular risk factors that differed mostly according to a history of pregnancy complications were BMI and lipid profile, with a higher rate of obesity and overweight and higher values of total cholesterol and LDL-C in women with a history of HDP and/or GDM. In addition, chronic hypertension was overrepresented in women with a history of HPD and/or GDM complicated pregnancies. Insulin resistance evaluated by means of HOMA–IR showed a similar trend.
It is well known that pregnancy complications exert their detrimental effects beyond the gestational period and a growing body of evidence supports long-term effects on maternal health and longevity [27]. Women with a history of HPD have an approximately doubled risk of CVD mortality (RR 2.81, 95% CI: 2.55–3.09) according to a recent systematic review and meta-analysis of 56 studies, including ischemic heart disease (RR 2.06, 95% CI: 1.38-3.08), heart failure (RR 2.53, 95% CI: 1.28–5.00) and stroke (RR 1.59, 95% CI: 1.08–2.33) [27]. The risk of chronic hypertension is five times higher in this population, with a peak within 5 years postpartum and a gradual decrease thereafter to a RR of 1.79 (95% CI: 1.22–2.61) beyond 15 years from delivery. A similar time trajectory was not evident for cardiovascular event risks, highlighting the importance of a long-term follow-up [28]. In addition, severity of HPD seems to modulate future cardiovascular risk, with early onset, severe and recurrent preeclampsia being associated with worse outcomes, in a “dose effect” relationship [29]. Also, GDM has been associated with a 2-fold increase in cardiovascular events (RR 1.98, 95% CI 1.57-2.50) in a meta-analysis of 9 studies involving over 5 million women, with the highest risk within the first decade postpartum [30]. The pathophysiological mechanisms underlying the association between APO and long-term maternal health are still a matter of discussion. Results from prospective studies involving large cohorts of women with a history of HPD suggest that the excessive cardiovascular risk is largely, but not completely, mediated by traditional cardiovascular risk factors, including hypertension, dyslipidemia, type 2 diabetes mellitus and increased BMI [31-33]. Similarly, CVD risk is present in women with a history of GDM, yet lower in those who do not develop a subsequent diagnosis of type 2 diabetes mellitus [30]. A diagnosis of diabetes type 2 is 10 times more frequent among women with a history of GDM [34]. On the other hand, subsequent development of hypertension and hyperlipidemia in women with a history of GDM has been inconsistently reported across studies [27,35]. That being so, it remains an unanswered question whether the excessive cardiovascular risk results from an underlying vulnerability and pre-existing traditional cardiovascular risk factors lowering the threshold for pathology manifestations both in pregnancy and later in life, or APO contributes to a persistent loss of functional reserve from a cardiometabolic point of view [36]. Indeed, pregnancy represents a “stress test” to the female body that has to adapt in order to achieve a balance between foetal development and maternal health, through hemodynamic and metabolic changes [37]. In women with underlying reduced functional reserve because of the interaction between genetic predisposition and adverse environmental exposure (e.g. stress, obesity, diet, comorbidities, inflammatory processes), which can be regarded as a “first hit”, pregnancy may represent a “second hit”, resulting in maladaptation and pathological modifications that may synergise with pre-existing risk factors in a long-term perspective of aging [38]. The reproductive fil rouge is in keeping with that idea that menopause may represent a “third hit” in this population of susceptible women, in which a maladaptive response to hypoestrogenism may induce cardiometabolic modifications [15].
The retrospective nature of this study does not allow evaluating changes in metabolic profile across the menopausal transition. However, our data showed that those modifications in cardiovascular risk factors typically observed around menopause (increased LDL-C and triglycerides, insulin resistance and increased BMI) [39] are more evident in women with a history of APO, in line with the hypothesis of a reduced cardiometabolic functional reserve in this population.
When we evaluated cardiovascular risk factors in menopausal women with a probable history of PCOS, we found a trend towards a less healthy metabolic profile as compared to the remnants; indeed, they were more likely to be hypertensive, overweight/obese and to have higher triglycerides. However, this association seems less robust than that observed for obstetric history; this may seem unexpected, since PCOS per se is a risk factor for pregnancy complications, including HDP and GDM [40], and several studies have shown increased risk of insulin resistance, dyslipidemia, obesity, hypertension and metabolic syndrome in this population [41]. On the other hand, current evidence suggests that PCOS phenotype ameliorates with aging and that the differences in cardiometabolic profiles between PCOS and non-PCOS women may lose their significance approaching the menopausal transition [42]. Data from longitudinal and cross-sectional studies including PCOS women older than 40 years reported a similar risk of hypertension, diabetes mellitus type 2 and cardiovascular morbidity and mortality compared to age and BMI matched healthy controls [43-46]. Contrasting information has been reported concerning BMI, with few but not all studies showing further deterioration of anthropometric measures after menopause, while hypertriglyceridemia emerged as the more frequent abnormality in lipid profile in menopausal PCOS women, in line with our higher non-significant data [47-50]. In our sample, a number of aspects may have hindered the assessment of PCOS impact at midlife on the evaluated cardiometabolic parameters. Firstly, we recognise the difficulty to define the postmenopausal phenotype of PCOS: diagnosis of PCOS in menopausal women is retrospective, based on a history of anovulatory cycles, clinical/biochemical hyperandrogenism and/or ultrasound PCOM [20]. However, these criteria may be poorly specific, especially when self-reported by women recalling characteristics of menstrual cycles from several years earlier. Moreover, it is difficult to differentiate retrospectively the different PCOS phenotypes (classic, ovulatory and non-androgenic phenotypes), which may represent a criterion to stratify patients according to metabolic risk, at least in women of childbearing age [51]. Indeed, it appears that young PCOS women with the classic phenotype are those showing worse cardiometabolic risk compared to the non-androgenic phenotype [51]. This observation may also provide a possible explanation of the excessive cardiometabolic risk dampening with aging, as androgen concentrations physiologically decrease from the fourth decade of life [52]. Finally, it is likely that a portion of menopausal PCOS women was excluded from our sample. Indeed, we only enrolled parous women, and it is known that PCOS is associated with infertility, particularly the classic phenotype, hence at higher metabolic risk [6]. Overall, although a history of PCOS should be considered when evaluating personal risk factors at midlife, it seems that a history of obstetric complication is more reliable as an alert for cardiometabolic health at every stage but especially at menopause.
Our study corroborates the idea that cardiometabolic health should be evaluated through a sex-oriented lens across women’s lifespan. Indeed, reproductive milestones are golden moments to assess risk factors and implement preventive or therapeutic strategies. Recent guidelines have been published in the attempt to standardise early lifestyle interventions and follow-up strategies starting from the postpartum to facilitate early detection and modification of cardiovascular risk factors [53,54]. Cardiology societies have included a history of pregnancy complications as a risk enhancer in the female cardiovascular risk assessments, but only postmenopausal women in the forthcoming years will benefit from this early prevention. For instance, the European Society of Cardiology have recommended following guidelines for secondary prevention in women with a history of HDP and GDM [5]. On the other hand, in the American College of Cardiology/American Heart Association guidelines for primary prevention of cardiovascular diseases, a history of HDP qualifies women at intermediate risk and supports the decision to start lipid lowering agents [55]. Implementation of such recommendations are fundamental to promote awareness about the link between APO and future health, which still appears low among both clinicians and patients [56].
Our study sample is representative of a population of women who are primarily referred to menopause clinics for the treatment of symptoms and prevention of related conditions, mostly at a relatively young age, before pathologies become manifested. This is probably the main reason why our women showed lower rates of obesity (14% vs 24.3% in 45-54 age group and 24.1% in the 55-64 age group), hypertension (22.5% vs 58.1%) and diabetes mellitus type 2 (2.2% vs 11.9%) compared to the Italian reference population [57]. Insulin resistance was a frequent finding, although we reported a discrepancy using HOMA-IR and TyG index as an estimate of insulin sensibility, with a higher percentage of women defined as insulin resistant using a TyG index cut-off of 8 compared to HOMA-IR cut-off of 2.5. This may be due to the relatively high percentage of overweight and obese women in the study sample, as TyG index seems to capture the metabolic risk more accurately in individuals with normal BMI [58]. Even the incidence of complicated pregnancy, including GDM (1.6%) and HDP (6.6%), was lower as compared to rates reported in the literature, being around 5% for GDM in Italy [59] and 10% for HPD worldwide [60]. Midlife women from our sample self-reported their obstetric history and missed cases could have been likely due to inaccurate recall. Moreover, as far as GDM is concerned, the relatively recent introduction of large-scale screening programs may have contributed to the low rate of the condition in our population who may experience their pregnancy few decades earlier. As an additional study limitation, data about waist circumference were not fully available from retrospective records; therefore, we were unable to define the prevalence of metabolic syndrome and the impact of reproductive history on it [20,21]. Finally, we acknowledge that our limited sample size, confined to parous women attending the clinic over 2 years, together with a low prevalence of reported APO, are an important limiting factor. Larger clinical samples are required in order to explore the role of reproductive history around menopause in cardiometabolic risk stratification.
Conclusions
Results of this observational study are in line with the evidence that obstetric history has a significant impact on women’s health later in life. Both pregnancy and menopause represent, through different mechanisms, “stress tests” which require adaptation and may unmask individual vulnerabilities. Women with a history of APO should be counselled early after pregnancy regarding their future cardiovascular risk and the need of modifying their risk factors and lifestyle. Despite the strong evidence advocating for a more vigorous early prevention in women with a history of APO, there is still no consensus on the best strategies to limit the excess risk in this population [29]. This poor preventive action in clinical practice possibly contributes to the lack of cultural awareness. Longitudinal studies aimed to identify clinical characteristics, biochemical and imaging markers predicting future risk in women with APO history, as well as specific therapeutic interventions for primary prevention, are highly auspicated to improve health of present new mothers and future menopausal women [61].
In conclusion, it seems that menopause consultation is a golden moment to outline the importance of obstetric history for future health, because related conditions might become manifest at this time. Therefore, preventive strategies need an early planning in the postpartum period, with periodic follow-up visits in order to modulate modifiable risk factors for a healthier woman at the time of menopause and beyond.
Acknowledgement
The authors are grateful to Professor Iuliana Ceausu (Department of Obstetrics and Gynecology I, "Carol Davila" University of Medicine and Pharmacy, "Dr. I. Cantacuzino" Clinical Hospital, Bucharest, Romania) that offered inspiration and supervision to Dr. Laura Cucinella as a member of the Junior Mentorship Program (JuMP) promoted by the European Society of Menopause and Andropause (EMAS).
Funding
Laura Cucinella and Rossella E. Nappi, as members of the European Campus of City-Universities (EC2U) Alliance (EC2U, Grant Agreement n. 101004065-EC2U), received funding at the University of Pavia, Italy, for the activities of Work Package 4 “Life-long wellbeing and healthy aging”.
Conflicts of interest
Rossella E. Nappi has on-going relationship with Astellas, Bayer HealthCare AG, Besins Healthcare, Exeltis, Fidia, Merck Sharpe & Dohme, Novo Nordisk, Shionogi Limited, Theramex, and Viatris. None of these entities is relevant to the present work. All the other authors have nothing to disclose.