Introduction
The utilization of autologous platelet-rich plasma (PRP) has surged in popularity across various clinical applications due to its potential to enhance tissue healing and regeneration. PRP is derived from peripheral blood by centrifugation [1]. After activation, platelets release their α-granule content, which is enriched with growth factors that induce cell proliferation, growth and differentiation [2]. The widespread adoption of PRP use in regenerative medicine has prompted clinicians to investigate its possible beneficial effects in premature ovarian insufficiency and decreased ovarian reserve. Small uncontrolled studies have reported that intra-ovarian autologous PRP injections increase ovarian reserve parameters resulting in increased mature oocyte yield, fertilization rate, formation of good quality embryos, as well as an increase in anti-Mullerian hormone (AMH) levels and a decrease in follicle-stimulating hormone (FSH) levels in previous non-responders [3-5].
Case report
A 39-year-old woman, gravida 4 para 0, was seen in the emergency department with suspected septic shock. She had a 3-day history of fever (39.5° C), headache, neck stiffness, oliguria, and abdominal pain. On presentation, vital signs were blood pressure of 107/73, heart rate of 96 beats per minute, and a respiratory rate of 20. Laboratory investigations showed a hemoglobin of 11.4 gr/dL, white blood cell count (WBC) of 25 x 109/L, and creatinine of 143 μmol/L.
The patient had undergone hysteroscopic intrauterine lysis of adhesions and bilateral ultrasound-guided transvaginal ovarian PRP injection for fertility treatment in a clinic outside of Canada without prophylactic antibiotics. Prior to the procedure, she had an AMH level of 2.2 pM/l and an antral follicle count (AFC) of 5. The patient started developing symptoms on post-procedure day seven. Pelvic ultrasound showed bilateral ovarian abscesses (7 cm on the left and 2 cm on the right), with multiple posterior myometrial cystic spaces and debris. The internal medicine team was consulted for her elevated creatinine and a history of venous thromboembolism with multiple previous bilateral pulmonary emboli, and the team suspected a possible meningitis. She had a lumbar puncture and a negative cerebrospinal fluid culture ruled out meningitis. She was admitted to the gynecology service for management of her bilateral ovarian abscesses.
The patient’s medical history was relevant due to four recurrent pregnancy losses (6.1-8.5 weeks), with the third loss complicated by deep vein thrombosis and multiple pulmonary emboli. Thrombophilia testing was negative in the past. Additionally, she had a two-year history of four perianal abscesses, which she managed through self-drainage at home. She had undergone a cone biopsy due to an abnormal Pap test. She had stage four endometriosis which was managed both medically and surgically in the past. Her surgical history included a hysteroscopic myomectomy, a hysteroscopic polypectomy, laparoscopic resection of advanced endometriosis and a mini laparotomy for the resection of a large distorting adenomyoma.
On admission, the patient was started on intravenous (IV) Ertapenem. She also received throughout her admission Enoxaparin for deep venous thrombosis prophylaxis. The acute kidney injury resolved with fluid resuscitation. An abdominal ultrasound did not identify renal pathology. Her blood cultures were positive for Escherichia coli and Staphylococcus epidermidis; the latter was thought to be a culture contamination. The infectious disease team was consulted to guide her antibiotic management, after which she was transitioned to IV Ceftriaxone, Metronidazole and Vancomycin. She had a percutaneous drain placed into her left ovarian abscess by the interventional radiology team. The patient became afebrile by post-admission day 4 and her WBC dropped to 11 x 109/L by post-admission day nine. Due to a transient increase in her abdominal pain after drain insertion, an abdominal CT scan was completed, which did not identify vascular or bowel injuries. Her pain subsided with oral and parenteral analgesics. Her ovarian abscess aspirate cultures were positive for Escherichia coli and Streptococcus Dysgalactiae. She was monitored in the hospital to ensure the antibiotics were well-tolerated due to her history of penicillin allergy. For transient pleuritic chest pain, she completed a chest CT scan which did not identify any pulmonary emboli, nevertheless, bilateral pleural effusions were seen. The patient was discharged home on post-admission day nine with an ovarian abscess drain bag and oral Amoxicillin-Clavulanate for 6 weeks. Multiple attempts by both the patient and members of her Canadian care team to reach the fertility center outside of Canada were unsuccessful. Updated AMH and further fertility treatment outcome after discharge are unknown.
Literature review
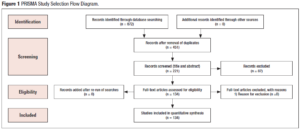
We aimed at reviewing the current literature and assess the safety of intra-ovarian PRP injections. For this we performed a thorough search of databases including MEDLINE, Embase, PubMed, Cochrane and Google Scholar using a search strategy developed by an Information Specialist (E.P.). The search used MeSH terms, keywords and Medical Subject Headings pertaining to platelet-rich plasma, PRP, autologous platelet rich plasma, ovarian insufficiency, premature ovarian insufficiency, decreased ovarian reserve, premature ovarian failure, premature menopause, in vitro fertilization, IVF, intracytoplasmic sperm injection, ICSI, Embryo transfer, endometriosis, endometrioma and low ovarian reserve. Figure 1 presents the PRISM study selection flow diagram.
Results
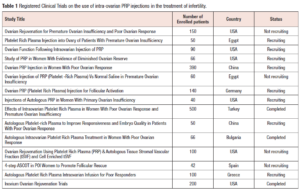
Of 134 studies that were reviewed, none were found that discussed the development of ovarian abscesses following intra-ovarian PRP injection. To the best of our knowledge this is the first case report of a bilateral ovarian abscess and sepsis after intra-ovarian PRP injection. There are no RCTs published on the use of intra-ovarian PRP injection, however, there are currently 15 registered RCTs around the world that are studying its use for the treatment of infertility with a total of 2,044 patients enrolled in these trials. Three trials have been completed (https://clinicaltrials.gov/) (Table 1).
A recent multi-center RCT of intraovarian PRP injection for women with poor ovarian response, presented as an abstract at the European Society of Human Reproduction and Embryology (ESHRE) 39th Annual Meeting, revealed that intra-ovarian PRP injection did not result in an increased number of oocytes retrieved [6]. A total of 83 patients, younger than 38 years of age, were randomized to the PRP group (n = 41) or the control group (n = 42). After treatment, patients underwent controlled ovarian hyperstimulation (COH), oocyte retrieval, intracytoplasmic sperm injection (ICSI), preimplantation genetic testing for aneuploidy (PGT-A), and single frozen euploid embryo transfer. Number of Metaphase II oocytes obtained was the primary outcome. No significant differences were observed in number of retrieved Metaphase II oocytes (3.1 ± 3.3 vs 2.8 ± 2.4 in PRP vs control group, respectively; p = 0.9) [6].
Discussion
Premature ovarian insufficiency (POI), is characterized by the loss of ovarian function before the age of 40 [7].7 This condition is marked by a decline in ovarian follicles leading to diminished hormone production, particularly estrogen. Consequently, those affected by POI experience irregular menstrual cycles, fertility problems, and hormonal imbalances as do woman approaching menopause at the more typical age [7]. The prevalence of POI was initially reported as 1-1.1% [7-9], but a more recent meta-analysis of the global prevalence of POI reported a pooled prevalence of 3.7% [10]. This could be partially explained by the increasing trend of late childbearing in developed countries, or improved record keeping, leading to an increase in the incidence of infertility caused by POI.
According to the European Society of Human Reproduction and Embryology (ESHRE), current treatment strategies to manage infertility associated with ovarian insufficiency are limited to in-vitro fertilization (IVF) treatment coupled with oocyte donation [11]. Outcomes are compromised in women of advanced reproductive age and/or with diminished ovarian reserve who utilize their own eggs, as no interventions have been consistently proven to enhance ovarian activity and rates of natural conception [11]. Additionally, certain cultures and religions prohibit egg donation which leaves those patients with limited options. In those who achieve spontaneous pregnancy after POI, the risk of obstetric complications is similar to that of the general population; however, those who receive oocyte donation are at high risk of obstetric complications, such as pregnancy induced hypertension and first trimester bleeding, and they should be closely followed in an obstetric unit [11,12].
A prospective preliminary report of intraovarian injections of PRP in extremely poor prognosis patients with only third-party oocyte donation as an alternative revealed no clinically significant effects on ovarian function or good quality embryo formation [13]. The protocol in this study included injection of non-activated PRP with up to 12 punctures per ovary, followed approximately one month later by a stimulated IVF cycle. Endocrine monitoring of 80 cases revealed no clinically significant effects on ovarian function, number of oocytes produced, or creation of good quality embryos. Two live born infants, however, were born to patients both 40 years of age with previous failed IVF cycles. As stated in the manuscript this outcome does not establish PRP as the underlying reason for these conceptions and additional trials are ongoing [13].
A recent publication from ESHRE reported that intrauterine and intraovarian PRP are not recommended outside strict research protocols in infertile women. Further investigation and well-designed studies are necessary to assess the efficacy and ensure the safety of this procedure before considering its use in routine clinical practice [14].
Despite the advances made in the treatment of infertility, those who receive a diagnosis of POI continue to face restricted treatment choices. To address this challenge, a novel wave of therapeutic methods is under development including: in vitro activation, mitochondrial activation technique, stem cell and exosomes therapy, biomaterials strategies, and PRP intra-ovarian infusion [7]. Nonetheless, these emerging therapies are currently in the experimental phase and demand meticulous research to expedite their transition into clinical application.
The application of PRP across various medical fields has demonstrated advantageous impacts on tissue regeneration, angiogenesis initiation, regulation of inflammation, as well as cell proliferation, migration, and differentiation [1,15,16]. PRP therapy exhibited the ability to enhance implant vascularization and the quality of autologous ovarian transplantation [17]. Additionally, intrauterine administration of PRP stimulated endometrial growth and enhanced pregnancy outcomes in patients with suboptimal endometrial lining [18]. While a few studies have explored the utilization of intra-ovarian PRP injection in patients with decreased ovarian reserve and POI, the potential for enhancing ovarian function through this approach has yet to be definitively established. The number of published complications in patients who underwent intra-ovarian PRP is limited, and there is insufficient literature on the impact and safety of PRP in managing infertility in patients with POI. Due to the lack of research on safety of PRP in patients with POI, and the lack of RCTs assessing its efficacy, its use remains experimental and is not approved by Health Canada.
The 39-year-old woman who presented to the emergency had a history of intrauterine adhesions and endometriosis, both of which are well established etiologies of infertility and recurrent pregnancy loss [19,20]. Endometriosis, defined as the presence of endometrial tissue outside of the uterine cavity, and consequential endometriomas (cystic lesions of the ovaries originating from endometrial glands and stroma) have been shown to decrease ovarian reserve, oocyte quality and embryos among affected individuals [21,22].
In addition to the risk factors that predisposed the patient to infertility, it is important to note that there were other factors that put her at high risk of infection. Aside from the PRP injection, she had undergone hysteroscopic intrauterine lysis of adhesions with no prophylactic antibiotics. Prophylactic administration of antibiotics before transcervical intrauterine procedures is not a standard practice as no RCTs have been published to evaluate the impact of prophylactic antibiotics on the incidence of post-procedure infections [23]. While Escherichia coli, Staphylococcus epidermidis and Streptococcus Dysgalactiae are not part of the normal vaginal flora, these microorganisms have been found as part of the human gastrointestinal flora [24] and may have been introduced into the ovarian tissue through perineal colonization or bowel injury.
Conclusions
Intra-ovarian PRP injection needs to be assessed on a larger scale in clinical trials with clear inclusion and exclusion criteria, technique, and follow-up to determine its safety and efficacy (if any) in female reproduction. Until then, autologous PRP ovarian treatment should not be recommended.
Declaration of interests
The authors declare having no conflicts of interest.