Introduction
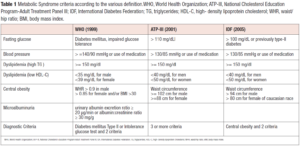
When discussing about the metabolic syndrome (MS), many specialists agree that it is has numerous causes; the most important of which is insulin resistance [1]. MS is defined according to specific criteria that are summarized in Table 1. As it can be easily argued, central obesity is the main common criteria among the presented classifications together with dyslipidemia and fasting glucose. Each of the classifications require the presence of 2 or more criteria at the same time to be present to state the presence of MS.
Obesity is defined by the WHO (World Health Organization) as a chronic illness characterized by an excess of fat accumulation that, over time, can lead to significant and severe diseases. There are two different kinds of obesity: visceral or central obesity (android) and peripheral obesity (gynoid). Android obesity, typical of men and postmenopausal women, is characterized by an increase in waist circumference, as measured using a waist/hip ratio (WHR) of more than 0.80. In gynoid obesity, which is typical in fertile women, fat is mainly localized in the thighs and buttocks. Because of the evolutionary function of the two genders, the distinct fat distribution reflects the diverse physical structure of men and females: men’s abdominal obesity allows hunting and escape, whereas gynoid fat distribution protects pregnancy in both mechanical and metabolic ways [2].
Things drastically change as we approach the menopausal transition. As a matter of fact, many women suffer weight gain and an increase in central adiposity in the years preceding the menopause. Central/visceral obesity is associated with important changes in lipid and glucose profiles, insulin resistance and/or diabetes mellitus, with an increase in metabolic and cardiovascular risk [3].
There are specific differences between central and the peripheral fat mass: the adipocytes of gynoid obesity have a higher insulin sensibility, and greater density of estrogenic receptors, thus promoting higher fat mass deposition than in the android fat cells. On the contrary, the latter are more sensitive to androgenic stimulation and produce and release a greater amount of pro-inflammatory adipocytokines into the portal circulation. The variable estrogenic or androgenic reactivity of fat mass cells, depending on the region of fat deposition, may explain why obesity localization differs across genders and throughout a woman's life [4].
Even in adults of normal weight, especially for women, there is a favorable link between increasing waist circumference and worsening cardiovascular profile [5]. Abdominal fat is a better predictor of the harmful impact of obesity on health than body mass index (BMI). According to criteria of the International Diabetes Federation (IDF), waist circumference is the main diagnostic feature of the MS, which inextricably links visceral adiposity with the worsening of the metabolic profile (Table 1).
MS and health issues
MS represents a complex disorder that correlates with significant mortality and morbidity rates and has high economic and social costs. It affects 20-25% of the general population, with an increasing frequency with age, particularly among individuals aged 50-60. The characteristics of the syndrome are visceral obesity, abnormal lipid and glucose metabolisms and high blood pressure. The specific pathophysiological feature is represented by insulin resistance, which may explain these metabolic impairments.
Insulin resistance promotes the storage of visceral fat tissue, which is less sensitive to insulin, and as a consequence causes a higher lipolytic function that may increase the release of non-esterified fatty acids (NEFAs) into liver circulation. Excess of NEFAs induces an abnormal production of glucose and triglycerides that impairs liver function and insulin clearance. Such findings were recently reported by Genazzani et al. [5] in individuals with polycystic ovary syndrome (PCOS). Indeed, PCOS individuals have been shown to have high rates of insulin resistance and obesity, as well as elevated ALT and AST levels at the top limits of normality [5]. This disorder is more common in PCOS individuals who have a family history of diabetes (i.e. diabetes in one or more parents and/or grandparents) and is associated with a higher Hepatic Insulin Extraction (HIE) index [5,6]. The increased HIE index shows that a significant amount of circulating insulin is due to reduced liver clearance and an extended half-life rather than pancreatic production. Moreover, fat mass should not be considered simply a “storage of energy” but an active “endocrine tissue” with no direct control, that releases adipocytokines with pro-inflammatory properties such as TNFα, IL-6, leptin, adiponectin. In addition, the decreased secretion of adiponectin triggers insulin resistance and elevates triglyceride and LDL cholesterol (LDL-C) levels [1,7].
If the MS begins during fertile life, or somewhat later, but before the initiation of the menopausal transition, women are more likely to experience health problems in the decades preceding menopause. Given that the menopausal transition and the beginning of menopause cause unique hormonal and metabolic changes, all of these events are added to the eventual impairments caused by the MS, which in turn increases cardiovascular disease (CVD) risk. Furthermore, given that the average life expectancy for women nowadays is close to 90 years, there is concern in terms of health prevention, especially if threatened by the combination of MS and aging/menopausal issues.
Obesity, menopausal transition and the MS
The menopausal transition starts approximately 8-10 years before the real onset of the menopause, identified as the lack of periods for at least 12 months in a row. During this transition there is a progressive increase in FSH levels due to the decrease in inhibin production by the ovaries. This change leads to a higher chance of presenting more anovulatory cycles, thus inducing a decrease in progesterone production during the luteal phase. All these events result in a relative hyperestrogenism, low progesterone, abnormal or rare bleedings followed by a long period of hypoestrogenism when the menopause has actually taken place [8].
To date, changes in body weight during the menopausal transition represent an important topic of discussion. It is obvious that weight changes are based on an impaired ratio between energetic input and energy consumption, the latter of which is determined by physical activity and resting energy expenditure. It is well known that women after the age of 45–50 years, in the absence of changes in their lifestyle (feeding and physical activity), show a progressive increase in body weight [9].
Even though feeding (i.e. food quantity), and mainly the quality of nutrients, represents a key factor for the maintenance of an optimal metabolic equilibrium, physical activity represents a fundamental element for the biological triggering of the metabolic pathways through energy consumption due to muscular function.
Weight gain during the menopausal transition is also linked to the progressive changes in the hormonal milieu up to the menopause onset. Menopause is associated with an increase in total body fat, and in particular abdominal fat [10]. Lovejoy et al. [11] have reported that during the perimenopause, abdominal fat mass increases inversely in correlation to the occurrence of menopausal hypoestrogenism, with minimal changes in body weight [11]. Interestingly, a reduced trend in total caloric intake has been observed during the menopausal transition [11].
Clinically, this scenario at the time of menopause is strongly influenced by the metabolic condition women had during their reproductive life. They may have suffered from metabolic disorders (overweight/obesity), including thyroid dysfunction or diabetes mellitus and/or insulin resistance, as in the case of PCOS. Being born as SGA (small for gestational age), suffered IUGR (intrauterine growth restriction), or having had a history of being overweight/obese during infancy and/or adolescence predisposes to a metabolic impairment that triggers insulin resistance. In other words, epigenetic variables can alter and exacerbate normal weight since infancy, and that personal health history, in general, affects the metabolic shift that occurs throughout the menopausal transition.
Gonadal steroid hormones have specific metabolic effects and these events are more evident at the moment of their decrease as soon as the menopausal transition begins. The menopausal transition is characterized by alternating hypoestrogenic and/or hyperestrogenic cycles with low progesterone levels until amenorrhea develops due to ovarian failure. Indeed, during the luteal phase of the menstrual cycle, the typical increase in P/E2 ratio induces an increase in body temperature of about 0.4°C [12]. The rise of temperature is due to the elevation of the basal metabolism of about 200 kJ (50 kcal) per day [12].
Decrease or absence of luteal progesterone, during the menopausal transition or when menopause occurs, determines a lack of energy consumption under resting conditions typical of the luteal phase (about 50 kcal a day, for 12–14 days: approximately 600–700 kcal every month). The lack of progesterone reduces consumption of energy, thus, triggering an increase in fat mass deposition during the perimenopause [14]; indeed, no luteal phase, hence no physiological burning of calories. This decline in energy consumption cannot be ascribed only to the progesterone deficiency. Also, other factors are involved such as the amount of lean mass at the moment of the menopausal transition, the activity of the sympathetic nervous system (SNS), the endocrine status and the aging-mediated physiological changes. The decline in basal metabolism, observed in postmenopausal women, also depends on aging [15]. However, during the menopausal transition there is also a significant decrease of basal metabolism, being this reduction greater than the observed during the aging process [16].
The decline of the metabolic functions is not due only to the lack of progesterone, but also to the hypoestrogenic state. As women shift from the “perimenopause” status to the “postmenopausal”, hypoestrogenism worsens insulin resistance, which is also triggered by the slow but progressive cortisol increase typical of aging. It is well known that cortisol induces gluconeogenesis and, this, further promotes insulin resistance. At the same level, hypoestrogenism also partly induces a significant reduction of growth hormone (GH) plasma levels, which predisposes to more storage of abdominal fat mass with a reduction in lipid metabolism [1,17]. Data on experimental animals have confirmed such observations, since estrogen administration in ovariectomized rats reduces fat mass and the adipocyte cell size, independently of the energy intake [18]. In addition, estrogens seem to accelerate fat oxidation pathways in muscles and adipocyte lipolysis [18].
As a consequence, it is obvious that a decrease in basal metabolism produces fat mass storage, which further contributes to the development of obesity and obesity-related disorders, particularly the worsening of insulin sensitivity, or insulin resistance, which is crucial for the onset of MS. Insulin sensitivity is critical because when it decreases, it causes the pancreas to generate more insulin in order to maintain glucose under control. A well-defined hepatic impairment has been observed in obese and/or overweight women with PCOS. In reality, the classic compensatory hyperinsulinemia caused by insulin resistance is characterized not only by an increased pancreatic output but also by a prolonged half-life due to impaired hepatic clearance of circulating insulin [6,19], as demonstrated by HIE index computation [19].
As a consequence, there is a greater incidence of MS in overweight/obese women during the menopausal transition, with a gradual worsening of the cardiovascular profile and a higher risk of glucose intolerance progressing to type II diabetes.
Obviously other factors might contribute to the weight gain during the menopausal transition such as orexin-A, which is a neuropeptide involved in the control of feeding behavior as well as in other neuroendocrine homeostatic functions [20-22]. Orexin-A shows higher plasma concentrations concomitantly to the hypoestrogenic condition of the menopause [23].
The approach to MS during menopause
It is important to approach menopause and menopausal transition considering all the clinical aspects that this peculiar moment represents for each woman. It is crucial to note that this period is very significant for each woman, and this is an important factor to bear in mind while explaining to each individual patient, what is happening during the menopausal transition, both in terms of hormonal and metabolic changes.
The importance of the anamnesis: recognizing the issue
The anamnestic evaluation has to explore the prenatal and/or postnatal history of the patient looking for the presence of perinatal issues (i.e. SGA, IUGR) or overweight/obesity during infancy and/or adolescence but also if there are any members of the family with diabetes or other endocrinological diseases and/or obesity. The purpose of this evaluation is to exclude any clinical cause that might require specific therapeutic approaches, such as any uncommon secondary obesities, due to genetics, neurological or psychiatric conditions.
Among other concerns to be considered is a history of PCOS and/or premenstrual syndrome and premenstrual dysphoric disorder (PMS-PMDD). Insulin resistance is a frequent feature of the PCOS, especially considering that 50–60% of the patients are overweight/obese [24]. In addition, it has been recently reported that patients with PCOS and/or insulin resistance have a double chance of suffering from PMS-PMDD and mood disorders, including up to depression during their reproductive life and during menopausal transition [25]. This condition is due to a lower production of progesterone and neurosteroids (i.e. allopregnanolone, the most powerful endogenous anxiolytic and anti-depressant substance derived from progesterone catabolism), responsible for the more frequent anovulatory cycles in PCOS patients [25]. Interestingly, allopregnanolone secretion is improved when insulin sensitizer treatment is administered to hyperinsulinemic PCOS women [26], thus, demonstrating that insulin resistance might participate in a greater occurrence of menopausal symptoms, as previously suggested [27].
Therefore, it comes clear that it is useful to assess the presence of insulin resistance, even among non-obese women premenopausal women, especially in those who suffered from PCOS and/or have had clinical evidences of genetic/epigenetic conditions that might have triggered a compensatory hyperinsulinemia. Non-obese women may have a normal weight thanks to their lifestyle even though they have a predisposition to insulin resistance, that could be disclosed evaluating insulinemia, after overnight fasting or, even better, performing an oral glucose tolerance test (OGTT). This test is usually carried out over 4 h, but a positive response to this test can equally be achieved with just two blood samples, at time 0 (i.e. before drinking the glucose) and 60 or 90 min after a glucose load of 75 g has been given. An hyperinsulinemic state is diagnosed when the insulin response is higher than 50-60 μU/ml [28,29]. Furthermore, in women with a history of PCOS during fertile life, reports indicate that insulin resistance has been demonstrated to still be present during both pre- and postmenopausal phase, since the response to OGTT resulted higher both for glucose and insulin in those subjects [30].
Therapeutic approaches
Therapeutical approaches for women who are in the transitional menopause stage is changing, and may depend on whether they are normal weight or overweight/obese. If body weight is within normal levels, the objective will be to avoid weight gain. In the case of overweight/obesity, it is critical to minimize additional weight gain and to identify and treat metabolic problems. In both cases, the first recommendation is to take care of lifestyle through the combination of a hypocaloric diet with physical activity. In general, 45 minutes of training (i.e. fast walking or jogging) three times per week is enough to maintain body weight control and induce fat mass loss, as well as to reduce waist circumference and blood pressure in overweight/obese women, in addition to decrease plasma glucose, triglyceride, and LDL-C levels while increasing HDL-C concentrations [31].
Recently Dupuit et al. [32], analyzed how changes in body composition may relate to overweight/obesity in postmenopausal women after moderate-intensity continuous training (MICT), high-intensity interval training (HIIT) and HIIT + resistance training (RT) programs. They showed that HIIT programs were more effective than MICT programs in reducing abdominal/visceral fat mass; whereas, MICT or HIIT + RT programs decreased weight and whole-body fat mass [32].
It is worth noting that hormone replacement treatment (HRT) protects against perimenopausal alterations in body composition in women with normal weight and climacteric symptoms [33]. In these women, no significant variations in weight, fat mass content and distribution have been observed, probably also thanks to HRT route of administration. Transdermal estrogens, in particular, appear to be more protective against fat mass growth and android fat distribution [34,35]. Similar effects on body composition and fat tissue have been observed when administering tibolone and raloxifene [35].
Obviously, lifestyle changes and/or the use of HRT are insufficient to manage overweight/obese women with the MS during menopausal transition. Specific pharmacological or integrative therapies, such as metformin, inositol, orlistat, and others, have shown to be beneficial in lowering insulin resistance and increasing insulin sensitivity, and may aid and facilitate weight reduction. Metformin, a well-known diabetic medication, lowers insulin levels while increasing insulin sensitivity causing an increased glucose cell absorption, and is traditionally given to hyperinsulinemic PCOS women during their reproductive years [36]. Also, the integrative approach has shown to be beneficial. Indeed, the administration of myo-inositol (MYO) alone [37], or in combination with D-chiro-inositol (DCI) and/or alpha lipoic acid (ALA) [38], seems to enhance insulin sensitivity through a post-receptor improvement. In addition, the integrative use of carnitines, L-arginine and N-acetyl cysteine has proved to be effective in improving insulin sensitivity in menopausal women by reducing the HIE index [19].
Recently, it has been suggested that the presence of familial diabetes (parents and/or grandparents with type I or II diabetes) greatly predisposes to insulin resistance, and to a compensatory hyperinsulinemia in PCOS patients due to a specific enzymatic impairment that affects MYO to DCI conversion and/or ALA synthesis inside the mitochondria [39-41]. Moreover, familial diabetes also predisposes to a reduced expression/function of the hepatic insulin degrading enzyme (IDE) that clears insulin from the blood circulation. Reduced IDE activity induces longer insulin half-life, facilitating hyperinsulinemia not sustained by pancreatic function [5,6,42]. In PCOS patients with familial diabetes, the use of the appropriate integration with DCI and/or ALA [43] seems to be more appropriate than the use of MYO alone [44], unless combined with ALA, since ALA allows a better liver function [45]. Interestingly, all PCOS patients that show insulin resistance, compensatory hyperinsulinemia and familial diabetes also show transaminase enzyme elevations (up to the upper limit of normality), being this a signal of liver impairment and a predisposition to NAFLD (nonalcoholic fat liver disease) [5,6,46]. In such cases, the use of ALA and/or a combination of ALA with DCI [5] or MYO [45] seems to be the appropriate integrative choice.
Because the majority of the above-mentioned impairments identified in PCOS patients are connected to genetic/epigenetic susceptibility, their occurrence during the menopausal transition cannot be ruled out. It is obvious that performing a thorough anamnestic history of the menopausal patient is very helpful in the selection of treatment. Indeed, biological susceptibility to metabolic abnormalities do not diminish while women transition from the fertile phase to the menopause.
In conclusion, the MS is greatly dependent from metabolic impairments and the most relevant one is insulin resistance. The latter is triggered by aging as well as by the reduced gonadal steroid synthesis due to the physiological ovarian failure occurring in the menopausal transition and after. When menopause occurs in patients that show familial diabetes and/or a history of PCOS, maybe also overweight/obesity, it is critical not just to improve life-style but also apply suitable integrative therapies, as with PCOS patients [44], eventually in combination with of metformin.
Conflicts of interest
The authors declare having no conflicts of interest.