Introduction
Due to the growth of the ageing population the problem of relieving symptoms caused by age-related changes in the female urogenital tract during menopause remains one of the most pressing problems in the field of gynecology. The term “genitourinary syndrome of menopause” (GSM) underlines the variety of genital, sexual and urinary symptoms associated with the anatomical and functional changes that occur in the vulvovaginal tissues during ageing [1].
According to the modern integrative definition, GSM is a complex of symptoms that includes physiological and anatomical changes that occur in the external genitalia, perineum, vagina, urethra and bladder secondary not only to estrogen deficiency but also to reductions in other sex steroids in women [2-4].
According to current recommendations on menopausal hormone therapy and health maintenance in older women, local estrogen therapy is the “gold” standard for the treatment of vulvovaginal atrophy. Moreover, local estrogen therapy in low doses is preferable for women with complaints of vaginal dryness or associated discomfort during sexual activity. Vaginal estrogens are generally more effective for alleviating urogenital symptoms than oral products, due to the absence of liver metabolism and a quick vaginal response [5,6]. The modern polyhormonal concept of GSM pathogenesis opens up new potential opportunities for studying ways to optimize traditional local estrogen therapy by additionally prescribing other sex hormone products in topical forms, for example, a combination of estriol with progesterone or androgens (dehydroepiandrosterone), thus turning local hormone monotherapy of GSM into combination local hormone therapy. As seen in clinical practice, complex hormonal exposure gives better results in a shorter time. After local repletion with estriol and progesterone, vaginal colonization with the necessary lactobacilli becomes more active, which is essential to restore normal vaginal microflora [7,8].
In Russia, besides estriol monoproducts, another product is used for intravaginal use in GSM, namely a combination of estriol with micronized progesterone and lactobacillus casei rhamnosus Doderlein (LCR) in vaginal capsules. Estrogens promote the growth and maturation of the vaginal epithelium, as well as the synthesis and accumulation of glycogen, which is a key substrate for the activity of lactobacilli and should be available in the lumen of the vagina in order to be utilized by lactobacilli. The process of release of glycogen from the vaginal epithelium depends on effects of progesterone, which contributes to the formation of intermediate layers of vaginal epithelium and its natural desquamation. A similar situation occurs in the hormone-dependent lower urinary tract, where estrogens perform the same critical physiological functions for the urothelium, ensuring its growth and maturation, the synthesis and accumulation of glycogen, as well as the synthesis of local immunity factors (immunoglobulins) and protective mucopolysaccharides – glycosaminoglycans (hyaluronic acid and its sodium and zinc salts, chondroitin sulfate, glycoproteins, mucin). They make up the surface glycocalyx of the bladder mucosa – a powerful natural system of antibacterial and anti-inflammatory protection of the lower urinary tract [9-11]. However, complete natural antibacterial protection of the urothelium of the urethra and bladder in women is impossible without progesterone, which is related to the fact that while estrogens affect the synthesis of glycosaminoglycans in the urothelium of the bladder, progesterone affects their release from the urothelium out into the lumen of the bladder [12,13]. Moreover, it is important to underline the following non-reproductive effects of micronized progesterone: analgesic, due to suppression of the synthesis of prostaglandins; neuroprotective and neuroreparative, due to a selective effect on the receptors of neurotransmitters; regulatory, due to increased synthesis of muscle protein [14].
Thus, recent data indicate that in order to ensure a normal anatomical and functional state of the lower urinary and genital tracts in women, sufficient levels of both estrogen and progesterone are essential [15-17]. A disorder of the synthesis and release of glycogen secondary to deficiency of sex hormones leads to rapid alkalization processes in the vagina and the development of dysbiotic processes – a decrease in the number of lactobacilli and excessive growth of pathogenic and opportunistic pathogenic microorganisms [18]. According to data from a detailed analysis of vaginal microflora of 73 postmenopausal women who did not receive hormonal therapy, 49% had no lactobacilli at all, while the others had the concentrations 10-100 times lower than in premenopausal women[19]). According to other studies, lactobacilli predominate in the vaginal microflora in only 13% of postmenopausal women not taking hormonal therapy. In postmenopausal women, the most common microorganisms are anaerobic gram-negative bacilli and gram-positive cocci [20]. Thus, the normalization of the vaginal microflora is one of the goals of treatment of GSM. Moreover, recently there has emerged evidence that normalization of vaginal microflora not only reduces the frequency of relapse of urinary tract infections in peri- and postmenopausal women, but also contributes to more rapid relief of GSM symptoms [21,22]. The purpose of lactobacilli in products for the treatment of GSM symptoms is to reduce pH and maintain normal biocenosis, preventing colonization of the vagina by pathogenic bacteria.
The goal of this study was to compare the efficacy, on GSM symptoms, of an intravaginal estriol monoproduct and a combination product containing estriol, micronized progesterone and LCR.
Materials and methods
An open comparative randomized prospective study was conducted at the clinical site of the Department of Obstetrics and Gynecology with a course of perinatology of the Peoples’ Friendship University of Russia (Moscow, Russia) in the period from 2017 to 2018, after examination and approval by the local ethics committee.
During the study, the following criteria for the inclusion/exclusion of patients were considered.
Inclusion criteria: women in early postmenopause (duration of postmenopause from 1 to 3 years) in accordance with the STRAW+10 criteria; a diagnosis of “postmenopausal atrophic vaginitis” (ICD No. 95.2) (GSM), confirmed by examination; sexually active (vaginal sex at least 2 times a month); negative Pap test; informed consent of patients to participate in the study.
Exclusion criteria: reproductive age; previous local or systemic menopausal hormone therapy; lack of signs of vaginal atrophy; hormone-dependent tumors; vaginal bleeding of unknown cause; malignant tumors of the reproductive system, as well as diseases of the vulva, cervix and vagina associated with HPV infection (intraepithelial neoplasia).
In accordance with the inclusion/exclusion criteria, 69 women who needed conservative treatment of GSM were enrolled in the study. The 69 patients were randomized into 2 groups and received only local hormone therapy. The patients in Group 1 (n=34) used intravaginal suppositories with estriol at a dose of 0.5 mg/day for 14 days, followed by a gradual dose reduction as symptoms improved until a maintenance dose of 1 suppository 2 times a week. The patients in Group 2 (n=35) used an intravaginal combination product in the form of capsules containing estriol (0.2 mg), micronized progesterone (2.0 mg) and a lyophilized culture of LCR (341 mg (2x107 colony-forming units)). The product was prescribed at a dose of 2 capsules once a day for 20 days, then 1 capsule a day. The total duration of therapy in both groups was 12 weeks.
The study consisted of two stages (treatment phase of 12 weeks and follow-up phase of 12 weeks) with three patient visits (Visit 0 – during the inclusion phase of the study, Visit 1 – 12 weeks after the start of therapy, and Visit 2 – 12 weeks after the end of therapy).
Clinical symptoms of GSM, both subjective (vaginal dryness, dyspareunia, irritation/burning/itching, dysuria, bleeding with sexual activity) and objective (relating to elasticity, vaginal folds, fluid secretion, epithelial thickness, moisture) were evaluated using the adapted Nappi RE scale; their severity was rated on a scale from 0 to 3 (0 – no symptoms, 1 – mild symptoms, 2 – moderate symptoms, 3 – severe symptoms) [4]. Subjective symptoms were evaluated by the patient, while objective symptoms were evaluated by the physician during the study visits.
All the patients underwent gynecological examination, where special attention was paid to the condition of the vulva, vaginal mucosa and cervix. The severity of symptoms of atrophic vulvovaginitis (dryness, burning/itching, dyspareunia, bleeding from the vagina after intercourse, recurrent discharge from the genital tract) was evaluated using the 5-point D. Barlow scale [23]. Vaginal pH value was also determined: a litmus indicator was inserted into the vagina with an exposure of 30 seconds, and the result was compared against a 3.0 – 7.0 pH color scale. Routine colpocytology allowed evaluation of the karyopyknotic index of the vaginal epithelium, defined as the percentage of surface cells with nuclear pycnosis to the total number of cells in a colpocytogram. Based on the gynecological and colposcopic examination as well as measurement of vaginal pH, Bachmann’s Vaginal Health Index was determined in points. Colposcopy was performed in all patients in order to rule out pathological processes of the cervix and verify epithelial atrophy of the vaginal walls. Hyperplastic and tumor processes of the cervix, uterus, fallopian tubes and ovaries were ruled out by sonography, pipelle biopsy (if needed) and Pap test.
The main software used for statistical analysis was the IBM SPSS 22 statistical package. Statistical analysis consisted of research and descriptive methods; it was conducted with the required power of the study of 80% (beta error of 20%) and an allowable alpha error of 5%. Demographic data of patients and baseline data were presented as rates or percentages, or using mean (standard deviation), median (interquartile range), minimum and maximum, depending on the type of variable. To test the homogeneity of the study groups at baseline, as well as after treatment, the null hypotheses (absence of differences between the groups) were tested using Student’s t-test (for interval indicators with normal distribution in the study population), the Mann-Whitney test (for ordinal indicators or for interval indicators with a distribution other than normal) or c2 test (for qualitative characteristics). In the presence of statistically significant differences between the groups, the magnitude of the differences between the groups was estimated.
Study results
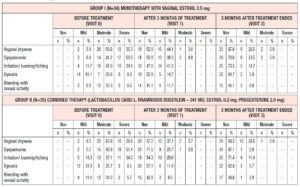
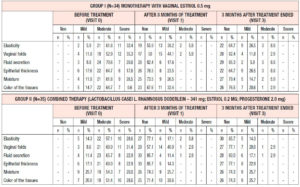
All the patients enrolled were under 60 years of age (mean age, 53.6 ± 2.1 years). The duration of postmenopause ranged from 1 to 3 years (2.04 ± 0.3 years on average), and the duration of GSM symptoms ranged from 1 to 3 years (1.4 ± 0.96 years on average). At the time of their visit to the doctor, no patient had been receiving systemic or local hormone therapy. At the time of enrollment in the study, all the patients had subjective and objective symptoms of GSM according to the Nappi RE scale of varying severity, without statistically significant differences between the groups (p>0.05) (Table 1).
Gynecological examination showed atrophic changes, of varying severity, in the labia majora and labia minora and perineum region. Vaginal mucosa in all patients was pale, thinned, smoothed, inelastic, sometimes with petechial hemorrhages, without natural moisture. No signs of inflammation or pathological discharge were detected.
When evaluating the severity of vaginal atrophy symptoms at the time of enrollment in the study using the 5-point D. Barlow scale, the values were found to range from 3.2 to 4.1 points. Measurements of vaginal pH indicated alkalization of the vaginal discharge; the average pH in the entire sample of patients was 6.3±0.4. Calculation of the karyopyknotic index during colpocytology showed low estrogen saturation of the vaginal wall, ranging from 28.2% to a maximum of 59.6%, while the average karyopyknotic index in the investigated population was 38±3.44%. Bachmann’s Vaginal Health Index averaged 2.15±0.98 points. It is important to note that the vaginal mucosa was easily injured when touched with an instrument to collect material for cytologic evaluation. Intergroup comparison of all the above initial patient data showed the absence of statistically significant differences, which indicates homogeneity of analyzed groups of patients with regard to the characteristics investigated (p>0.05).
Gynecological examination, including with the use of a colposcope, after 12 weeks of therapy, revealed, in almost all the women in the study population, significant improvements in the condition of the skin and mucous membrane of the vulva and vagina, which had a pale pink color and sufficient moisture. There was also a subjective decrease in complaints of itching, burning, dryness and dyspareunia in the patients; they showed increased elasticity, new normal epithelium, and no longer presented petechial hemorrhages on the vaginal mucosa.
Efficacy of therapy was analyzed in the entire the population of patients enrolled in the study at 12 weeks after the beginning of treatment. Persistence of the achieved therapeutic effects was evaluated 24 weeks after the beginning of therapy (12 weeks after the end of treatment); this evaluation was based on the severity of the objective and subjective symptoms of GSM according to the adapted Nappi RE scale.
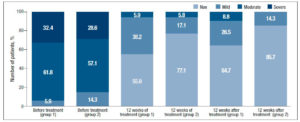
Analysis of subjective symptoms showed that after 12 weeks of therapy (Visit 1), dyspareunia was alleviated in 18 (52.9%) patients in Group 1 and in 25 (71.5%) in Group 2, p<0.05. An interesting fact was that 12 weeks after the end of therapy (Visit 2), further improvement was observed in both groups with regard to relief of dyspareunia, which differed between the groups: in Group 1, the symptoms subsided completely in 24 (70.6%) patients, i.e. they persisted in 30% of cases, while in Group 2 the symptoms subsided completely in 34 (97.1%) patients, p<0.05 (Figure 1). With regard to the remaining subjective symptoms of GSM (vaginal dryness, irritation/burning/itching, dysuria, bleeding with sexual activity), there were no statistically significant differences in the response to the treatment and during follow-up (p>0.05) (Table 2).
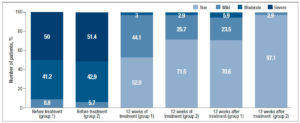
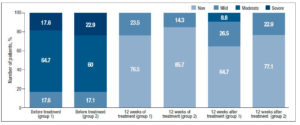
When evaluating the objective symptoms of GSM, a number of statistically significant differences were observed between the groups. Improved elasticity after 12 weeks of therapy (Visit 1) was observed in 19 (55.9%) and 27 (77.1%) patients in Group 1 and Group 2, respectively (p<0.05); normal epithelial thickness was observed in 26 (76.5%) and 30 (85.7%) patients, respectively (p<0.05). Moreover, 12 weeks after the end of treatment (Visit 2), improved elasticity was observed in 22 (64.7%) and 30 (85.7%) patients in Group 1 and Group 2 (Figure 2); normal epithelial thickness was observed in 22 (64.7%) and 27 (77.1%) patients, respectively (p<0.05), (Figure 3). As for the remaining analyzed objective symptoms (relating to vaginal folds, fluid secretion, moisture, color of the tissues), there were no statistically significant differences in the response to the treatment and during follow-up (p>0.05) (Table 3).
Finally, with regard to the rest of the analyzed parameters (D. Barlow scale, pH, Bachmann’s Vaginal Health Index), statistical analysis did not reveal any statistically significant differences between the groups. When evaluating the severity of symptoms of vaginal atrophy on the 5-point D. Barlow scale after 3 months of therapy, the values varied ranged from 1.8 to 2.4 points. Three months after the beginning of the therapy, pH values reached an average of 4.1 ± 0.3 and Bachmann’s Vaginal Health Index was 4.65±0.59 points. The colposcopy results showed that the degree of epithelial atrophy corresponded to 1.25±0.12 and 1.45±0.16 points; no abnormal colposcopic symptoms (acetowhite epithelium, punctation, mosaics) were observed after treatment.
Discussion
During our study we observed, on the basis of certain subjective and objective signs (dyspareunia, elasticity and epithelial thickness), that combined estrogen-progesterone local hormone therapy with probiotic support is a more effective method of treating GSM than estriol monotherapy. The differences observed and the long-term effect on dyspareunia are likely to be associated with increased proliferative processes, improved blood supply, and restoration of microbiocenosis when using combination therapy for GSM.
Recommendations of The National Institute for Health and Care Excellence (NICE) published in November 2015 (Menopause: diagnosis and management) confirm that not all menopausal problems are related to vasomotor symptoms; in particular, the problem of vulvovaginal atrophy was highlighted. Even without the use of systemic menopausal hormone therapy, local estrogen therapy by itself is quite effective for relieving symptoms and treating GSM [24,25].
The document states that local vaginal estrogens can be used for as long as necessary. These recommendations also indicate that there is no risk of endometrial hyperplasia and, therefore, that there is no need to monitor the endometrium or additionally prescribe progesterone to protect the endometrium when using topical estrogen products. Other scientific documents, including a review of the Cochrane Database, came to similar conclusions [26-29]. There are also data showing no negative effects of local estrogens on breast cancer or thrombotic risks [30].
Although monohormone estrogen therapy for dealing with vulvovaginal atrophy might be seen as a logical concept, it is obvious that the genitals, all the structures of the lower urinary tract, as well as the musculofascial apparatus of the pelvic floor, and the urothelium and endothelium of the indicated anatomical regions contain receptors for all sex steroids: estrogens, progesterone and androgens [31-33].
Despite the fact that guidelines state that there is no need to include progestogens (natural progesterone or its analogs) in topical estriol therapy, the new definition of GSM and its polyhormonal concept allows it to be claimed that progesterone might be an important component of the treatment of urogenital disorders, in order to obtain a complete effect [21]. A decrease in the proportion of lactobacilli due to both a deficiency of estrogens and a deficiency of progesterone and associated disruption of synthesis and release of glycogen exacerbates the process of vaginal atrophy.
The use of combination estriol-progesterone therapy with a probiotic for GSM treatment is not only appropriate, but advisable since there are receptors for all sex steroids in the vagina, urethra, bladder and pelvic floor muscles. Progesterone effects on tissues are no less important in restoring their physiological state.
Conclusion
The main limitation of our study is its small population. Despite this limitation, this is the first study showing not only significant positive and long-term effects, but also advantages of a combination local hormonal therapy containing microdoses of estriol, micronized progesterone and LCR on some subjective and objective symptoms of GSM (especially dyspareunia) compared with estriol monotherapy.
Conflict of interest
The authors declare that there is no conflict of interest.