Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophin-related polypeptide member of a family of growth factors involved in the development and preservation of the central and peripheral nervous system. BDNF interacts with high-affinity tyrosine kinase receptors (TrkB) and with the unselective p75NGF receptor (1){Tapia-Arancibia, 2004 #83}. BDNF is expressed in several areas of the adult rat, monkey and human central nervous system (CNS) (2), where it plays a significant role in the outgrowth, differentiation and survival of central and peripheral neurons during development as well as in adulthood (3). Experimental studies in rats showed that BDNF is involved in long-term potentiation in the hippocampus (4, 5), in protection against apoptotic cell death (6), and in the promotion of activity-dependent dendritic growth (7), and consequently plays an essential role in age-related changes in learning and memory (8).
The fact that, in mammals, BDNF concentration is highest in the hippocampus, hypothalamus and parietal and frontal cerebral cortex supports its involvement in cognitive processes (9, 10). In this regard, it has also been demonstrated, using cerebellar Purkinje cells, that BDNF is one of the mediating factors of dendritic growth; therefore, the decline of this neurotrophin is associated with age-related neuronal atrophy and death; this decline occurs for instance with ageing or in some neurological disorders (11, 12).
Additionally, current studies in rats show that BDNF in plasma and the CNS is largely dependent on gender, age and hormonal milieu (13). Age-related changes in BDNF mRNA expression and protein, together with changes in its receptors, are found in the hippocampus and hypothalamus of rats (11). Significant increases in hypothalamic BDNF mRNA and protein levels were found during the first postnatal week, these increased levels remained stable during the adult stage and subsequently decreased in old rats (13).
It is now accepted that in rats and humans (13, 14) BDNF is strictly related to hormonal status; moreover, previous studies demonstrated that an estrogen-response element is located in the BDNF gene, suggesting that estrogen can regulate BDNF expression (15). In agreement with this hypothesis, gonadectomy has been shown to decrease BDNF mRNA and protein levels in the rat hippocampus and, to a lower extent, in other brain areas and in plasma; conversely, estrogen treatment can restore them. Also, the lowest levels of BDNF mRNA and protein were detected in rats during the afternoon of proestrus when the highest levels of progesterone are present (8).
In humans of both sexes, age-related changes in plasma BDNF levels have been correlated with cognitive decline, impairment of libido and wellbeing, and the risk of neurodegenerative diseases; however, hormone replacement counteracts these symptoms, improving quality of life (14). In parallel, the growth, differentiation, normal physiology and ageing of the CNS are all now recognized to be influenced by gonadal steroid hormones. Dehydroepiandrosterone (DHEA) and its sulphate ester, DHEAS, are the most abundant steroid hormones in the human body. However, their physiological significance, their mechanisms of action and their possible roles in diseases remain to be defined in different tissues. DHEA and DHEAS concentrations in humans typically decrease steadily with age, approaching a nadir at about the time many diseases of ageing become more prevalent. Observations such as these, coupled with basic and preclinical demonstrations of the biological effects of DHEA, fostered hope that restoring DHEA to youthful levels might, conservatively, increase well-being and, optimistically, extend life, protect the brain, ameliorate sex function, and delay the ravages of ageing, as reported by Maninger et al. (16). In view of these data, the present experimental study aims to investigate possible variations in BDNF levels in selected brain areas and in plasma after the administration of different doses of DHEA in female ovariectomized (OVX) rats.
Animal model and the study protocol
A total of 60 healthy female Wistar rats (weight 150-200g) purchased from Harlan Nossan (Milan, Italy) were used in the present study. Fertile rats were divided into six groups (N=10 rats in each group). For the acclimatization period (14 days), the rats were housed all together in a climate-controlled room; they had 14 hours of illumination (light on at 6 a.m. and light off at 8 p.m.). Food and water were freely available. Bilateral ovariectomy was performed under tiletamine plus zolazepam anaesthesia (Zoletil, 1 mg/rat IM), with the only exception being the rats in the fertile control group. Rats were OVX at the same estrous cycle stage, as indicated by vaginal smear (evaluated daily). Oral treatment was started 2 weeks after surgery. The animals were treated with estradiol valerate (E2V, 0.05 mg/kg/per day), and with different doses of DHEA (1, 2 and 5 mg/kg/per day). These doses were chosen because they are almost comparable with those administered in human clinical practice. One group of 10 fertile and one group of 10 OVX rats were used as controls; specifically, they did not receive active treatment. All treatments lasted 14 days, a time that, considering the life expectancy of rats, has been demonstrated to be sufficient to determine neuroendocrine milieu changes in these animals. Twenty-four hours after the last dose of treatment, each animal was killed by decapitation, after which the following were carefully dissected: frontal and parietal lobe, hypothalamus and hippocampus. All tissues were weighed. A blood specimen was immediately drawn from each rat after decapitation and collected in heparinized plastic tubes. In each tissue and plasma sample, BDNF was measured with an enzyme-linked immunosorbent assay (ELISA) method. Animal care, maintenance and surgeries were conducted in accordance with Italian law (D.L. n. 116/1992) and European legislation (EEC n. 86/609). The study was approved by the ethics committee of the Medical Faculty of the University of Pisa.
BDNF assay
Levels of BDNF in plasma and brain samples were determined with an ELISA method (BDNF Emax Immunoassay System, Promega, USA), after appropriate dilution of samples (1:4) using Block & Sample Buffer, and after acidification of brain tissue samples according to the manufacturer's instructions. Briefly, 96-well flat-bottom Immuno plates (Iwaki, Japan) were coated with an anti-BDNF monoclonal antibody and incubated at 4° C overnight. After blocking by non-specific binding with Block & Sample Buffer, standards and samples were added to the plates and incubated and shaken for 2 hours at room temperature. Subsequently, after washing with TBST wash buffer, plates were incubated for 2 h with anti-human BDNF polyclonal antibody. The last incubation required the addition of anti-immunoglobulin Y-horse radish peroxidize conjugate. In the last step of the assay, TMB One solution was added to develop the colour. After stopping the reaction with HCL 1N, the absorbance was read at 450 nm on a microplate reader and BDNF concentration was determined automatically according to the BDNF standard curve (ranging from 7.8 to 500 pg ml-1 purified BDNF).
The entire procedure was performed using a semi-automated Basic Radim Immunoassay Operator (BRIO, Radim, Italy) equipped with a microplate reader of optical density. A computer system linked to the BRIO analyzed the final results and expressed them in picograms per millilitre for plasma, and picograms per milligram of tissue for tissue samples.
Parameters used and statistical analysis
Plasma BDNF levels are expressed in pg/ml; tissue BDNF levels are expressed in pg/mg tissue. All data are reported as mean ± standard deviation (SD). Data were analyzed using Graph Pad Prism Software version 5.0 for Mac (Graph Pad Software, San Diego, CA, USA). The data obtained were analyzed by one-way analysis of variance, as appropriate, and the Bonferroni multiple comparison tests was used to compare treatment groups. Values of p < 0.05 indicate significant differences between the groups.
Results
Ovariectomy
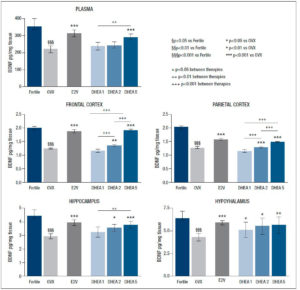
As expected, ovariectomy considerably reduced BDNF levels in all the examined tissues (p<0.001) and in plasma (p<0.001).
Effects of estradiol valerate (0.05 mg/kg/day)
After E2V administration, BDNF levels rose significantly in all analysed areas (p<0.001), and in plasma (p<0.001), reaching levels in the range of fertile controls in the frontal and parietal lobes.
Effects of DHEA (1, 2, 5 mg/kg/day)
Administration of DHEA at the dose of 1 mg/kg/day did not positively affect the reduction/decrease of BDNF related to ovariectomy that was seen in all brain areas and in plasma. Instead, DHEA enhanced BDNF content in the hypothalamus (p<0.05), in the hippocampus (p<0.05), and more significantly in the frontal and parietal lobes (p<0.01 and p<0.001 respectively) when administrated at the dose of 2 mg/kg/day. Administration of DHEA at the highest dose (5 mg/kg/day) increased BDNF levels in all analysed areas and plasma (p<0.001), reaching levels similar to those observed in the fertile group in the frontal and in the parietal lobes. The results are reported in detail in figure 1.
Discussion
In the present experimental study on a rat model, gonadectomy was found to reduce CNS and circulating levels of BDNF in fertile rats, while the administration of 0.05 mg/kg/day of E2V increased BDNF in all the analysed areas and in plasma, thus confirming a plausible link between estrogen and BDNF.
Previous studies demonstrated co-localization of estrogen receptors and the neurotrophin receptors p75NGF and TkB in the adult basal forebrain, suggesting an interaction between estrogens and neurotrophins (17-19). Moreover, the BDNF gene has been shown to contain an estrogen-response element (15), proving a direct relationship between estrogens and neurotrophins; furthermore, estrogen regulates BDNF gene expression in a developmental stage- and brain region-specific manner (20-22). Previous research has confirmed the importance of the estrogenic milieu in the function of basal forebrain cholinergic neurons and the trophic influences of BDNF on the cholinergic system (23). The main findings of our investigation are that DHEA administration at (2 and 5 mg/kg day is effective in restoring BDNF levels in selective brain regions in OVX rats and that this efficacy shows a dose-dependent trend, with significant differences between different doses. Specifically, the administration of DHEA 1 mg/kg/day did not affect neurotrophic tone in any of the analysed areas while the administration of DHEA 2 mg/kg/day markedly enhanced BDNF levels in the parietal and frontal lobes, less so in the hypothalamus and the hippocampus, and showed no effect on plasma BDNF concentration. The administration of DHEA at the highest dose (5 mg/kg/day) strikingly increased BDNF levels in the hypothalamus, in the hippocampus and in plasma, as well as in the frontal and parietal lobes where DHEA produced an even greater increase in BDNF, which reached levels similar to those observed in the fertile group.
In vitro and in vivo documented effects of DHEA involve neuroprotection, neurite growth, neurogenesis and neuronal survival, apoptosis, catecholamine synthesis and secretion, and also include anti-oxidant, anti-inflammatory and anti-glucocorticoid effects (24). Although there is still debate on DHEA receptors, these findings corroborate the evidence that DHEA is not just a pre-hormone of the adrenals, but rather a hormone in its own right, and that its metabolites modulate a series of biological processes, with a remarkable tropism for the CNS. Also, DHEA and its principal circulating metabolite, DHEA-sulphate (DHEA-S), have been demonstrated to exert a large range of direct effects on neuronal function (25-27). The relationship between DHEA and neurotrophic tone for the maintenance of neuroendocrine homeostasis is also confirmed in rodent experimental models. It is interesting to note that stress and ageing processes differently modify neurotrophin expression, especially BDNF expression (1); in particular, stress rapidly increases BDNF mRNA and protein levels in the hippocampus, hypothalamus and pituitary, the principal structures of the HPA axis (28-30). In recent decades, it has been widely demonstrated that central administration of exogenous BDNF induces significant modifications in HPA axis activity, thus confirming the involvement of BDNF in the HPA setting (28).
DHEA serves as a precursor for estrogens and androgens from fetal life to post-menopause, and many people believe that DHEA is merely an inactive precursor pool for the formation of bioactive steroid hormones. In the brain, DHEA is a neurosteroid that acts as a modulator of neurotransmitter receptors, such as gamma-aminobutyric acid type A, N-methyl-D-aspartate, and sigma-1 receptors (25, 26). In addition, DHEA-S may also exert effects through its more immediate metabolites, such as 7α-hydroxy-DHEA (31). Higher concentrations of DHEA are found in the brain in comparison with plasma values, with a brain-to-plasma ratio of ~6.5. As mentioned above, In vitro and in vivo specific effects of DHEA involve neuroprotection, neurogenesis and neuronal survival, apoptosis, catecholamine synthesis and secretion, and also include anti-oxidant, anti-inflammatory and anti-glucocorticoid effects (24); although DHEA is not produced and secreted by rat adrenal glands in the same amount as in humans, high DHEA levels in rat brain derive from local synthesis, supporting the biological plausibility of preclinical neuroendocrine studies (32).
It is interesting to note that, like neurotrophins, levels of neurosteroids, especially DHEA (33, 34) in aged rats are decreased in the CNS (35). Our experimental evidence, in accordance with previously reported central effects on rats, corroborates the hypothesis of a strong relationship between DHEA supplementation and a neurotrophic milieu designed for HPA axis regulation as well as for modulation of the neuroendocrine setting both in physiological and in pathological conditions.
It is known that in humans DHEA serves as a precursor for estrogens and androgens. To this extent, oral supplementation of DHEA in postmenopausal women results in the formation of significant amounts of 17b-estradiol and estrone, accompanied by increases in androstenedione, testosterone and dihydrotestosterone. This, plus the evidence that DHEA can also be converted into estrogens and other androgens within cells, supports the view that many actions of this steroid are indirect and mediated via estrogen and/or androgen receptors (36-40). However, the rate of DHEA metabolism into estrogen/testosterone in different tissues, the presence of enzyme regulators, and the effect of the ageing process on the intracrinology of DHEA require additional investigation.
It is mandatory to consider that, unlike what is seen in most animal models used in the laboratory, where the secretion of sex steroids takes place exclusively in the gonads with no significant amount excreted by the adrenals (41), in humans peripheral target tissues can transform the adrenal precursor steroids DHEA-S and DHEA into androgens and/or estrogens (31). Analyses of sulphated steroids have also confirmed high concentrations of DHEA-S and pregnenolone sulphate in rodent and human brains (32, 42). Humans and rodents differ in the pathways through which sex steroids are synthesised. Whereas DHEA-S is the most abundant circulating steroid hormone in the human body (43), rats and mice have low circulating concentrations of DHEA-S in the periphery (44-46). Brain DHEA in the rat is derived from local synthesis and not from peripheral synthesis. In humans, brain DHEA concentration is the result of both local synthesis and peripheral synthesis.
In conclusion, our experimental study demonstrated that DHEA administration can protect female OVX rats from the effects of castration, restoring central and peripheral BDNF levels in a dose-dependent manner. Further studies are mandatory to establish possible biomolecular mechanisms underlying this process.
Acknowledgments: None
Conflict of interest: The authors declare that there is no conflict of interest