INTRODUCTION
Osteoporosis is a chronic non-communicable disease that requires long-term treatment. One main reason for this is that age is a crucial risk factor for the condition,1 therefore, once treatment is prescribed, the indication persists and is reinforced as the individual grows older. A tailored selection of the available anti-osteoporotic drugs will have to be applied, taking into account important features like efficacy, mechanism of action, and short- and long-term safety. For example, it has been advised that bisphosphonates, a first-line option in several guidelines, should be interrupted once they have been used for a number of years2,3. Consequently, the sequential treatment design, which proposes the ordered concatenation of drugs with different actions on bone metabolism, has come to light. The aim is to get the most from each drug in terms of bone accrual and also benefits, if any, at the level of other organs. The popularity of sequential combinations is reflected in an increasing body of literature on the topic4-8.
Sequential therapy regimes are particularly suitable in the case of women with osteoporosis diagnosed soon after menopause, because the relatively young age of these women means that they will require a long-term strategy. The Spanish Menopausal Society (AEEM) issued a clinical practice guideline supporting the sequential use of selective estrogen receptor modulators (SERMs) followed by bisphosphonates9. While acknowledging the lack of protection against hip fracture, the rationale for proposing SERMs as a primary option was based on several arguments. The first was the protection offered by SERMs against vertebral fracture, which, until women reach advanced age, is far more prevalent than hip fracture10; a further argument concerned additional benefits in terms of health, including the reduction of invasive breast cancer, and possibly — this is still debated —, cardiovascular disease risk 11-13.
Although obviously interesting from the perspective of women’s health, the main difficulty with that sequential therapy proposal is the lack of data on its impact on key areas, bone and others. We here present a pioneering study in which women who had been treated with raloxifene for 2 years were offered the chance to continue with raloxifene or to change to monthly ibandronate, a bisphosphonate. The impact of switching to ibandronate vs prolonging raloxifene treatment was investigated both at bone level and in relation to several metabolic targets, specifically lipids and insulin resistance.
METHODS
Subjects
Caucasian postmenopausal women, aged 50-69 years, with osteoporosis at the lumbar spine and/or femoral neck [bone mineral density (BMD) measurements corresponding to a T-score of -2.5 or lower] were considered eligible and invited to participate in the study. Menopause was defined by at least 1 year of amenorrhea and a follicle-stimulating hormone (FSH) level greater than 40 U/L. According to their medical records, a basic examination, and a routine serum analysis, the women were healthy.
Exclusion criteria were: current thyroid or parathyroid disease, diabetes mellitus, elevated transaminases, impaired renal function, hyper- or hypocalcemia, use of oral glucocorticoids, previous bone fragility fractures, or any metabolic bone disease that could interfere with interpretation of the findings. Women were also excluded if they had ever received medication for osteoporosis other than calcium and/or vitamin D.
All subjects provided written informed consent before undergoing any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice Guidelines, and the protocol was approved by the Ethics Committee of our center after assent from AEMPS (Spanish Agency of Medicines and Medical Devices).
Study design
This was a prospective, open-label study in which two distinct phases were designed. The first phase consisted of a 24-month period in which the participants were treated with raloxifene (60 mg/day, Evista®, Daiichi Sankyo Europe GmbH, Germany). The second phase consisted of 12 months in which women were given the option to continue with raloxifene therapy or to change to 150 mg/month ibandronate (Bonviva®, Roche, UK). A supplement of 600 mg elemental calcium plus 400 IU vitamin D daily was maintained across the two phases of the study.
Study procedures
The women were studied in accordance with the protocol usually followed at our center. Briefly, the baseline visit was followed by subsequent checks performed at the 3rd, 6th, and 12th month during the first year of treatment, and annually thereafter.
At each visit, anamnesis was performed, together with basic clinical and analytical explorations. Height, weight, waist circumference, and blood pressure were measured and blood samples obtained. BMD [lumbar spine and hip, dual energy x-ray absorptiometry (DXA)] was measured at baseline, and then annually over the two phases of the study.
Adherence to treatment was checked at each visit based upon the information provided by the women. Adverse events were also recorded at each visit.
Biochemical measurements
Women were admitted to the outpatient center of our hospital after an overnight fast, and blood was drawn between 8:00 and 10:00 a.m.; the blood was allowed to clot, and the serum was separated and kept at -80º C until analysis.
Glucose (mg/dl), a total lipid profile [total cholesterol (TC, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL), and triglycerides (TG, mg/dL)], creatinine (mg/dl), phosphate (P, mg/dL) and total calcium (Ca, mg/dL) were assayed by routine methods using an autoanalyzer (Olympus 5400; Tokyo, Japan). Hormonal determinations including 17β-estradiol (E2, pg/mL), intact parathyroid hormone (PTH, pg/mL), FSH (U/L), 25 (OH) vitamin D (ng/mL), thyroid-stimulating hormone (TSH, μU/mL), insulin (μU/mL), and levels of C-terminal telopeptide (CTX, ng/ml), were measured by electrochemiluminescence (E170 Modular Analyser; Roche Diagnostics, Mannheim, Germany).
Insulin resistance was assessed by the homeostasis model assessment (HOMA) from fasting glucose and insulin concentrations. HOMA-IR was defined according to the formula: fasting plasma glucose (mmol/L)×fasting insulin (μU/mL)/22.514. Insulin resistance was defined as HOMA-IR values ≥ 2.5.
Bone mineral density
BMD (g/cm2) was evaluated by DXA at the femoral neck and at the lumbar spine (L2-L4) using a Lunar DPX densitometer (GE Medicals Systems LUNAR Corporation, Madison, WI, USA). The coefficient of variation was 1% for the spine and 1.2% for the femoral neck. The Z- and the T-scores, which reflect the number of S.D.s by which a given measurement differs from the mean for a sex- and age-matched population and for a normal young adult reference population, respectively, were calculated.
Statistical analysis
Descriptive statistics were used to summarize the subjects’ characteristics at baseline. The significance of longitudinal changes in the parameters was determined by one-way analysis of variance (ANOVA) with repeated measures. Data were compared between the 2 groups by Wilcoxon’s signed rank test. Unless otherwise specified, data are expressed as median with ± 95% confidence interval (CI). Differences were considered significant if < 0.05. The statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), v. 17.0 for Windows.
RESULTS
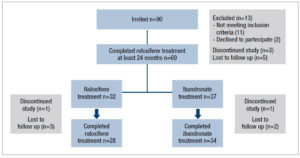
A total of 90 women with densitometric osteoporosis were invited to enter the study. Of these, 77 were considered eligible according to the inclusion and exclusion criteria and were prescribed raloxifene 60 mg/day for 2 years. Figure 1 shows the flowchart of the study. The 69 women who completed phase 1 of the study (2-year raloxifene treatment) were offered the opportunity to continue with raloxifene or to switch to ibandronate. The women opting for ibandronate cited the one pill/month regime as the main reason for deciding to change.
Thirty-two of the 69 women who completed phase 1 opted to continue with raloxifene while the other 37 women decided to change to ibandronate. Of these 69, the final analysis included 28 (raloxifene) and 34 (ibandronate) women (Figure 1).
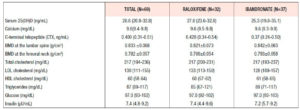
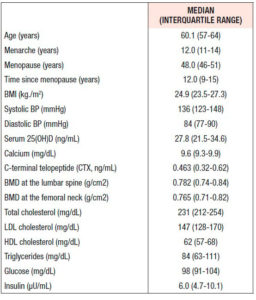
Table 1 shows the clinical and analytical features of the whole cohort at baseline. The subjects had a median age of 60.1 years, with an average of 12.0 years since menopause. Not shown in the table, there was no significant difference in any parameter between the women once they were divided into subgroups according to the treatment arm joined in the second phase of the study. Median BMD T-scores at the femoral neck and lumbar spine were -1.31 (±0.69) and -2.60 (±0.61), respectively.
Thirteen women (18.8%) were smokers and 17 (24.6%) did no form of physical activity. Body mass index (kg/m2) was normal (≥18.5 and <25) in 35 women; one woman was underweight (<18.5), 26 women were overweight (≥25 and <30), and 7 women were obese (≥30).
Phase 1 - raloxifene
Bone effect
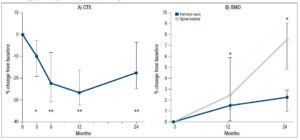
The intake of raloxifene for 2 years had a significant effect on different bone parameters (Fig 2). Specifically, the serum CTX values decreased significantly from baseline, with median percentage change of -10.4% (95% CI: -19.3, -2.9; p<0.05), -22.3% (95% CI: -30,9%, -8.4%; p<0.01), -26.8% (95% CI: -32.1,-16.3; p<0.01) and -17.6% (95% CI: -24.8, -3.7; p<0.01) at 3, 6, 12 and 24 months, respectively (Fig 2A). The calcium levels remained within the normal range throughout the study.
Median spine BMD increased significantly from baseline both at month 12 (2.4%; 95%: CI: 1.1, 5.8; p<0.05) and month 24 (7.5%; 95% CI: 4.8, 9.0; p<0.05) (Fig 2B). Median femoral neck BMD also showed increases from baseline at month 12 (1.5%; 95% CI: 0.1, 2.5) and month 24 (2.2%, 95% CI: 0.9, 2.9), but these changes, more modest, were not statistically significant. (Fig. 2B).
Lipid profile
Serum lipids showed beneficial changes. Serum TC decreased significantly at 3, 6, 12 and 24 months, with median percentage changes of -5.6% (95% CI: -8.7, -2.5; p<0.01), -7.4% (95% CI: -10.4, -4.9; p<0.001), -8.2% (95% CI: -11.9, -5.8; p<0.001) and -6.5% (95% CI: -9.2, -4.1; p<0.01), respectively. Concomitant decreases were found in LDL-C, which showed median percentage changes of -8.1% (95% CI: -12.8, -4.0; p<0.001), -10.7% (95% CI: -14.2, -7.1; p<0.001), -12.1% (95% CI: -18.4, -2.7; p<0.01) and -9.4% (95% CI: -13.3, -6.4; p<0.01) at 3, 6, 12 and 24 months, respectively. HDL-C increased significantly only at month 6 (9.53%, 95% CI: 4.3, 14.8; p<0.01). There were no significant changes in serum levels of TG.
Metabolic changes
The levels of fasting glucose remained unaltered, whereas insulin showed a significant reduction of 12.2% (95% CI: -24.7, +0.3; p<0.01), which determined a concomitant HOMA-IR decrease of 11.7% ( 95% CI: -25.4, +2.0; p <0.01) at month 6.
No significant changes in FSH, E2, TSH, PTH, or vitamin D were observed over the 2-year period.
Phase 2 - raloxifene vs ibandronate
Table 2 shows the bone and analytical values on completion of the 2-year raloxifene treatment (phase 1). No difference was detected between the study arms.
Bone effect
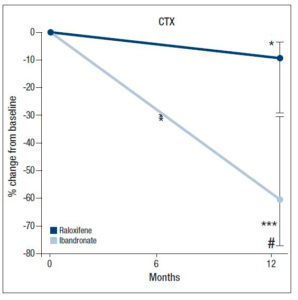
In phase 2, the values of CTX declined further in both groups, but the changes were more sizeable in the ibandronate group, which recorded a median percentage change of -65.5% (95% CI: -77.4, -33.4; p<0.001) vs −10.8% (95% CI: -31.7, -3.0; p<0.05) for raloxifene. This decrease in CTX was significantly different between the two groups (p<0.01; Fig. 3).
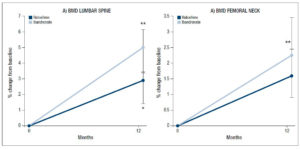
BMD continued to increase at the lumbar spine in both groups, but the median 5.0% (95% CI: 3.4, 6.1, p<0.01) increase recorded in the subjects who switched to ibandronate was higher than the median 2.9% increase (95% CI: 1.5, 3.4, p<0.05) observed in the subjects who continued with raloxifene (Fig. 4A). The difference between the groups, however, was not statistically significant.
Increases, albeit more modest ones, were also found in BMD at the femoral neck. The median increase recorded by the ibandronate group (2.3%; 95% CI: 1.6, 3.4) was greater than that found in the patients on raloxifene (1.6%; 95% CI: 0.7, 2.3), but the difference was not significant (Fig. 4B).
Lipid profile
The lipid parameters remained unaltered in the group continuing with raloxifene. In contrast, a significant increase in LDL-C (7.6%; 95% CI: 2.8, 17.2) was observed after 12 months of treatment with ibandronate (p<0.05). No significant difference, however, was found when LDL-C values at 12 months were compared between the two groups. No detectable change in HDL-C or TG was observed in the ibandronate group.
Hormonal changes
No significant change was detected in any parameter in the group continuing with raloxifene. In contrast, insulin levels increased under ibandronate (11.2%; 95% CI: 4.3, 17.2; p<0.025) and the HOMA-IR score increased (18.2%; 95% CI: 6.2, 25.9; p<0.025). However, no significant difference between the two groups was found. Moreover, the percentage of patients with HOMA-IR > 2.5% (insulin-resistant) was greater in the ibandronate group (35.3%) than in the raloxifene group (30.8%). No other significant changes were observed in any of the other hormones.
DISCUSSION
We found that treatment with ibandronate, a bisphosphonate, further reduced the bone resorption rate of women previously treated with raloxifene. This was confirmed by the clear changes in bone turnover, as shown by the levels of CTX. These women also showed increased BMD at the spine and, to a lesser degree, at the hip, but these changes were not significantly different from those recorded in the group of patients continuing with raloxifene. It is possible that larger groups might have clarified the differences. These data confirm the higher anti-resorptive power of ibandronate, in comparison with raloxifene.
But our study provides other information of interest, too. Indeed, the multi-target idiosyncrasy of SERMs raises questions of how the benefits at other organs or systems may be affected by the treatment change. In that regard, two important areas to consider are the lipid profile and insulin resistance.
According to a previous RCT, raloxifene induces beneficial lipid changes, mainly reductions in TC and LDL-C15. In line with those observations, both TC and LDL-C decreased under raloxifene in our study; during the year under ibandronate therapy, this effect faded progressively, particularly for LDL-C.
As regards insulin sensitivity, the effect of raloxifene is debated. While some investigators have found that raloxifene had no effect on insulin sensitivity or glucose tolerance in normal postmenopausal women16,17, others have found a slight18, or significant reduction in insulin sensitivity19. We only detected an isolated beneficial change in both insulin levels and the HOMA-IR but, interestingly, the switch to ibandronate led to an increase in the HOMA-IR, as though the beneficial effect had been reverted.
To our knowledge, this is the first study investigating this particular sequential treatment combination. The findings of this study are very relevant for the debate on whether SERMs should be the first choice after hormonal therapy, when used in the treatment of osteoporotic women who are still sufficiently young to expect to need a long-term treatment. The advantages of SERMs in such women are multiple. First, SERMs are a family of anti-resorptives that operate through the estrogen receptor (ER) intracellular mechanisms; from that point of view, SERMs seem particularly suitable for tackling postmenopausal osteoporosis, whose main trigger is the dramatic fall of estrogens at menopause. Second, the anti-resorptive potential of SERMs is lower than that of other anti-resorptives like bisphosphonates; this limits the power of action of SERMs but, in turn, frees them of some of the side effects associated with stronger anti-resorptives20-22. Again, this feature may be particularly advantageous for younger women, who are strong candidates for long- term treatment. Third, SERMs are multi-target compounds, which interact with ERs in whichever tissue. Given their ability to act as an agonist/antagonist depending on the compound and the target tissue, they may bring important health benefits in terms of reduced risk of breast cancer12 and, possibly, cardiovascular disease13. And fourth, the protection offered by SERMs against vertebral fractures has been demonstrated to be competitive, even when compared with bisphosphonates23. This is a strong argument when considering younger patients, whose main risk is vertebral fracture as opposed hip fracture, whose incidence instead peaks at advanced age.
Taken together, the above arguments provide the rationale for a sequential proposal, such as that reported in our study, in which SERMs may be used for a number of years. Then, when the risk of hip fracture becomes significant, SERMs may be replaced by a stronger anti-resorptive. The bone changes induced by ibandronate in our participants, as shown by both bone turnover and BMD data, confirmed its stronger anti-resorptive potential.
In conclusion, we found that the replacement of raloxifene with ibandronate was followed by an additional reduction in bone turnover. The reduction in LDL-C obtained with raloxifene was lost after one year. There might also be a worsening of insulin sensitivity as a consequence of the switch to ibandronate. These changes may have considerable clinical significance. Our conclusions, however, are limited by the small size of our cohort and by the observational nature of the study design.
CONFLICT OF INTEREST STATEMENT
None declared.