INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age 1,2. Yet, due to the numerous different phenotypes, its etiology remains unclear3. It seems, however, that endocrine disrupting chemicals (EDCs) can play a crucial role in the pathogenesis as they can impact the release, action, and metabolism of endogenous hormones. Thus, they can also be involved in modulation of the clinical severity of PCOS4.
Bisphenol A (BPA) is one of the most common plasticizers, present in a variety of objects of daily use, such as food packages, cans, electronic equipment, dental sealant materials, carbonless receipts, eye lenses, and water pipes 5. On account of its estrogenic properties, BPA is classified as an EDC or endocrine disruptor (ED), which means that it “alters function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or (sub)populations” 6. The impact of BPA on a variety of cells, via classical signaling (estrogen receptors: ERα and ERβ) as well as via non-classical pathways, has been confirmed and widely described 7-10. BPA can be released as a monomer from the aforementioned objects; hence, humans are constantly exposed to its endocrine disrupting properties from early life, with the fetus exposed to BPA in the amniotic fluid and postnatal exposure coming in a variety of forms, such as milk, plastic toys, plastic bottles, electronic equipment, food packages and cans, leading to increased serum BPA concentrations11. It is thought that this prolonged exposure to low doses of BPA may promote adverse health effects. A negative impact of BPA on human health has been reported in relation to reproductive disorders, infertility, miscarriages, prenatal development, and metabolic disorders including obesity, type 2 diabetes, coronary heart disease and hormone-dependent cancers 12-23. Moreover, recent studies have highlighted its potential role in the pathogenesis of PCOS 24-26. Hypothalamic BPA exposure may activate the gonadotropin-releasing hormone (GnRH) pulse generator, which in turn may lead to increased luteinizing hormone (LH) and decreased follicle-stimulating hormone (FSH) secretion by the pituitary, and therefore promote ovarian hyperandrogenism. BPA can also be involved in direct stimulation of androgen production in the ovarian theca cells leading to hyperandrogenemia and subsequent hyperestrogenemia. Women with PCOS have been found to present higher concentrations of BPA in biological fluids 26,27 and, in premenopausal women, serum BPA levels correlated with hepatic steatosis and markers of low-grade inflammation 28.
The aim of this study was to analyze serum BPA concentrations using high pressure liquid chromatography combined with tandem mass spectrometry (HPLC-MS/MS). Our goal was to identify the potential impact of BPA on the hormonal profiles of women with and without PCOS, in order to discover whether this ED may be an environmental factor in PCOS pathogenesis.
SUBJECTS AND METHODS
In total, 79 women aged 17-40 years were enrolled in the study. Thirty-five were diagnosed with PCOS according to the ESHRE/ASRM consensus (Rotterdam Criteria), and therefore showed two out of the following three features: clinical or laboratory indices of androgen excess; chronic anovulation; the presence of polycystic ovarian morphology visible on transvaginal ultrasonography. The control group consisted of 44 women without any endocrinopathy and not taking any hormonal contraceptives. All the women were recruited at the Department and Clinic of Endocrinology, Diabetology and Isotope Therapy, Medical University of Wrocław. Serum concentrations of LH, FSH and 17β-estradiol were analyzed. Serum levels of thyroid-stimulating hormone (TSH), prolactin (PRL) and 17-OH-progesterone were measured in order to exclude hypothyroidism, hyperprolactinemia and non-classical congenital adrenal hyperplasia 29. Total serum testosterone (TST), dehydroepiandrosterone sulfate (DHEA-S), and sex hormone-binding globulin (SHBG) were also measured, in order to evaluate the extent of hyperandrogenemia and to calculate the free androgen index (FAI) according to the formula: TST/SHGB*100% 30. All the aforementioned hormone and protein analyses were performed in certified, accredited clinical laboratories using immunoassay methods such as radioimmunoassays (RIA) (DIAsource ImmunoAssays, Belgium) or in the Wrocław laboratory using the chemiluminescent method (IMMULITE 2000 by Siemens Healthcare, Erlangen, Germany). Blood for the analysis of serum BPA concentrations was collected from all the participants in the morning after an overnight fast at the follicular phase of the menstrual cycle. After centrifuging the blood samples at 2500 rpm for 15 min, they were isolated and the serum was stored using BPA-free equipment. The study was performed in accordance with the guidelines of the 1964 Helsinki Declaration on human experimentation and with local university ethics committee permission. Informed consent was obtained from all the participants. We analyzed serum BPA concentrations using HPLC-MS/MS at the Department of Analytical Chemistry of the University of Technology (Gdańsk, Poland).
Sample preparation
Five hundred µL of solution of BPA-d16 in acetonitrile (can) (200 ng/mL) was added to 500 µL of human serum sample. Than 1000 µL of acetonitrile (ACN) was transferred to sample for protein precipitation. The obtained solution was vortexed for 30 s. Afterwards, 250 mg of magnesium sulphate (MgSO4) was added to the solution, which was vortexed again and centrifuged at 6000 rpm for 2 min. The supernatants were collected and transferred to glass test-tubes. These were placed in a water bath at 42˚C and evaporation was obtained under a gentle stream of nitrogen to approximately 150 µL. The residue was mixed with 250 µL of water, placed in a sample vial and analyzed.
The standards of BPA (≥99%) and deuterated BPA-d16 (98% D) were purchased from Sigma-Aldrich (Deisenhofen, Germany), LC-MS grade ACN from Merck (Darmstadt, Germany) and anhydrous MgSO4 from Eurochem BGD (Tarnów, Poland). Ultrapure water was obtained with the use of an laboratory HLP 5 system (device fits the ISO 3696:1999 standard for the first, second and third purity classes) from Hydrolab (Wiślina, Poland). The chromatographic separation was carried out using an HPLC system (Shimadzu, Japan) consisting of a degasser, binary pump, autosampler and a column oven. The analytes were separated on Lichrospher C18 column (Merck, Darmstadt, Germany; 250 mm × 4 mm; 5 µm). Elution was accomplished with an isocratic mixture of ACN:water 1:1 v/v. The mobile phase flow rate was maintained at 1 mL/min; the temperature of the column compartment was set at 45˚C while the injection volume was set at 10 μL. All analyses were done using a Shimadzu LCMS-8050 triple quadrupole mass spectrometer (Shimadzu, Japan) equipped with an electrospray ionisation (ESI) source working in the negative MRM (Multivariate and Repeated Measures ) mode. The transitions of ions monitored were 227.0> 211.9 for BPA and 240.0 > 142.2 for BPA-d16.
Serum BPA analyses
BPA concentrations were determined using LC-MS/MS. The Shimadzu triple quadrupole LC-MS/MS system (LCMS-8060; Shimadzu, Japan) was used for the analyses. Full details of the method are published elsewhere 31. LC-MS/MS was chosen in order to provide a high sensitivity method that is additionally believed to be a selective and accurate means of determining phenol concentrations 32. The limit of quantification was 0.028 ng/mL and the limit of detection was 0.0093 ng/mL.
All calculations were performed using the computer program STATISTICA 13.2 for Windows (Tibco Software). The normality of data distribution was tested using the Shapiro-Wilk test. To compare data with non-normal distribution, the Mann-Whitney U test was performed. To compare intra- and inter-group differences, two-way ANOVA was used. P values less than 0.05 were considered statistically significant.
RESULTS
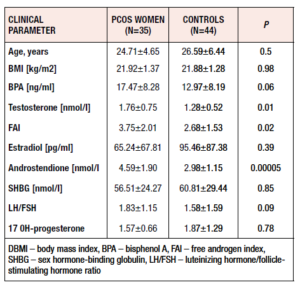
We studied 79 women – 44 healthy controls (mean age: 24.71±4.65 years) and 35 patients with PCOS (mean age: 26.59±6.44 years). All the study participants were age- and BMI-matched. Women with PCOS had significantly higher TST (p=0.01), FAI (p=0.02) and LH/FSH ratio (p=0.09) values. Table 1 presents the clinical and hormonal characteristics of both groups.
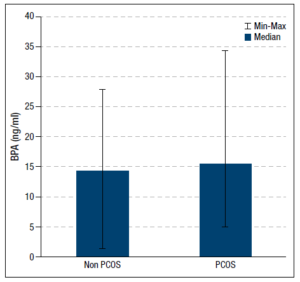
We detected BPA concentrations in 77% (n=34/44) of sera samples from the healthy women and in 60% (n=21/35) of those from patients with PCOS. The group with PCOS had the higher serum BPA concentration (p =0.06). Figure 1 compares the BPA levels of the two groups. Correlations between serum BPA concentrations and single hormone levels were investigated only in women with BPA detected in their serum samples. After dividing the participants into those with and without detectable serum BPA, no differences in the clinical and hormonal characteristics were found between these subgroups.
We did not observe any correlations between BPA level and hormonal profile in the group of healthy women. However, such associations were found in the PCOS patients; specifically, we observed positive correlations between serum BPA level and TST (r=0.53, p=0.05), 17β-estradiol (r=0.58, p=0.029), LH/FSH ratio (r=0.58, p=0.03), FAI (r=0.68, p=0.007), and androstenedione (r=0.61, p=0.02). Moreover, serum BPA also negatively correlated with serum SHBG (r=-0.48, p=0.08) in this group.
DISCUSSION
PCOS, the most common endocrine disorder in women, is characterized by ovulatory dysfunction and hyperandrogenism. The prevalence of PCOS may be correlated with the progress of civilization, with industrialization and with the plasticization of daily life. Many data have supported the thesis that environmental factors play an important role in the pathogenesis of PCOS4,25,33-35.
One of the most common plasticizers in daily life is BPA, which is produced all around the world in huge quantities (millions of tons every year) 36. Given the widespread use of BPA and the fact that it can be released as a monomer from everyday objects, humans are constantly exposed to it. This exposure begins prenatally, via the amniotic fluid of pregnant women exposed to BPA, then continues throughout postnatal life via different sources of exposure: milk, plastic toys, plastic bottles, electronic equipment, food packages and cans 11. This prolonged exposure, even to low doses of BPA, may promote adverse health effects 37-41. The considerable problem of human exposure to BPA and its potential health consequences has been addressed by the European Safety Food Authority (EFSA). In January 2015 the safe level of tolerable daily intake (TDI) of BPA was reduced from 50 micrograms per kilogram of body weight per day (µg/kg of bw/day) to a temporary TDI of 4 µg/kg of bw/day. The EFSA has now appointed a new working group of scientific experts that started evaluating recent toxicological data on food contact materials containing BPA in 2019. In 2020 they will re-assess the potential hazards of BPA and review the temporary safe level set in the EFSA’s previous full risk assessment 42.
It has recently been postulated that exposure to BPA may play a role in the pathogenesis of PCOS 24. On the basis of published data, we suggested that BPA can disrupt the hormonal profile and influence PCOS phenotype via different pathways. Briefly, we hypothesized that hypothalamic BPA exposure may activate the GnRH pulse generator, which in turn may lead to increased LH and decreased FSH secretion by the pituitary, and therefore promote ovarian hyperandrogenism. Furthermore, we supposed that BPA can also be involved in direct stimulation of androgen production in the ovarian theca cells leading to hyperandrogenemia and subsequent hyperestrogenemia.
Given the small number of studies that have directly examined serum concentrations of BPA in relation to the pathogenesis of PCOS, the results of our preliminary work provide valuable data that may explain some possible pathogenetic mechanisms of this disorder.
We detected measurable levels of BPA in sera samples from healthy women and from patients with PCOS. This observation confirms the widespread exposure of women to this plasticizer, which is similar to that observed in other civilized countries 43. Analogous to the research of Kandaraki et al. 44 and Konieczna et al. 26, our data also showed a higher concentration of BPA in sera from women with PCOS compared with healthy controls. This correlation may be a consequence of higher androgen levels in PCOS patients, as different mechanisms linking these aspects have recently been proposed. First of all, BPA may directly stimulate ovarian theca cells to produce androgens 45. Additionally, some data have suggested that BPA may displace endogenous hormones from SHBG binding sites, and thus disrupt the androgen-estrogen balance 46. Our results may support this thesis as we, and others 26,27, have found a positive correlation between serum BPA concentration and TST and FAI in women with PCOS, leading to hyperandrogenemia and subsequent hyperestrogenemia. Furthermore, our work, like that of others 47, also suggests that decreased SHBG levels are the result of increased serum concentration of BPA in this group, which may further increase levels of free androgens and BPA.
On the other hand, a correlation between androgen metabolism and BPA clearance has also been described. The conversion of androgen by P450 cytochrome may be suppressed by BPA 48, and elevated levels of androgens seem to inhibit the clearance of BPA by down-regulation of the liver enzyme uridine diphosphate-glucuronosyl transferase activity 49,50. These findings may explain higher concentrations of BPA in serum from men as well as women with PCOS 49,51,52. If these data are confirmed in further investigations, it would be worth finding a way of reducing exposure to BPA, especially in women with PCOS, in order to protect them from consequences of hyperandrogenemia. On the other hand, the disrupted BPA clearance may be the effect of BPA-induced hepatotoxicity 47,53.
The positive correlation between serum BPA and elevated LH/FSH ratio that we found in women with PCOS may support our first hypothesis of a BPA impact on GnRH pulsatility. It seems to be a very important finding as women with PCOS are clinically described with increased GnRH pulse frequency, resulting in increased LH and decreased FSH levels 54,55. Although the mechanisms of this neuroendocrine abnormality are still not well understood, it is well known that increased LH stimulates ovarian androgen production 55 and decreased FSH impairs follicular development and leads to anovulation 56. Elevated androgen levels impair the GnRH pulse generator, exacerbating all these negative effects. Data from animal studies support our results and describe an impact of BPA on the GnRH pulse frequency 57-60.
Summarizing, PCOS is a complex, multifactorial endocrinopathy that may be influenced by exposure to the EDCs present in daily life. Our preliminary study indicates that BPA can disrupt hormonal profile in PCOS women, but no such results were observed in healthy women. It supports the thesis that BPA may be a potential environmental factor involved in the pathogenesis of PCOS. The influence of BPA on hyperandrogenemia in PCOS women – probably via direct stimulation of the ovaries, release of androgens from SHBG, and increased LH stimulation – possibly exacerbates the “vicious cycle” of disruption of hormonal balance and BPA clearance in women with this syndrome. Further, carefully designed studies are needed to evaluate this suggested role of BPA in the pathogenesis of PCOS.
DECLARATION OF INTEREST
Authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING
The research conducted for this manuscript was partially supported by the own funding of the Department of Analytical Chemistry, Faculty of Chemistry, Gdańsk University.