Introduction
Oocyte cryopreservation has long been a challenging procedure in ART settings due to the poor efficiency of the slow freezing technique1. The advent of the vitrification method, with very fast cooling rates preventing ice formation, has led to a remarkable increase in the efficiency of oocyte cryopreservation2, which is now a safe and consistent technique3, offering outcomes comparable to those seen in fresh cycles 4-8.
These successful results have paved the way for a range of approaches in different assisted reproduction settings, such as female fertility preservation for both medical and non-medical reasons, oocyte banking in oocyte donation settings, or cycle pausing in cases of sperm sample collection impossibility. Finally, oocyte vitrification also offers an alternative approach for the treatment of women with poor ovarian response scheduled to undergo preimplantation genetic testing for aneuploidies (PGT-A).
A direct correlation between the success rate after a PGT-A cycle and the number of embryos available for selection has been extensively demonstrated9-11. It is relatively frequent in PGT-A cycles to have a low number of embryos for genetic analysis, and the number of mature oocytes retrieved is one of the major conditioning factors. Several causes that result in a poor response to ovarian stimulation have been identified: genetic, surgical, and, the most frequent one, the woman’s advanced reproductive age.
Moreover, it is widely known that advanced reproductive age is also associated with increased oocyte aneuploidies12,13 and therefore increased embryo aneuploidy14. The low number of embryos available for analysis and the high percentage of abnormal embryos account for the limited success of PGT-A in women of advanced reproductive age. Furthermore, despite a lack of consensus, some authors have also reported increased chromosomal abnormalities in embryos from young women with poor ovarian reserve15-17.
In PGT-A cycles with oocyte accumulation, two or more successive oocyte retrieval cycles with vitrification are proposed in order to accumulate an adequate number of oocytes. It is expected that a final cycle including both the fresh and the vitrified oocytes will yield a greater number of embryos for analysis18,19. However, there is very little information regarding the efficiency of this approach20. Data obtained from oocyte accumulation experience is very valuable not only to test the strategy itself, but also to assess the efficiency of oocyte vitrification in poor prognosis patients as, to date, outcomes of vitrified oocytes have been reported mostly in young good-prognosis patients.
In this regard, the objective of this study was to assess the impact of oocyte vitrification on embryo development, euploidy rate and implantation in patients undergoing cycles of oocyte accumulation for PGT-A. The clinical outcomes of the PGT-A cycles with oocyte accumulation were also analysed in order to validate the proposed strategy.
Materials and Methods
Study design
This is a retrospective study of 95 PGT-A cycles with oocyte accumulation by means of vitrification performed between January 2010 and March 2013. During that period, PGT-A was performed by means of D3 biopsy, array-CGH analysis and fresh embryo transfer.
The indications for PGT-A were recurrent implantation failure (n=26, 27.4%), severe male factor (n=26, 27.4%), recurrent miscarriage (n=22, 23.1%), and advanced maternal age (n=21, 22.1%).
Patients with <10 metaphase II (MII) oocytes retrieved in the first treatment cycle were advised to vitrify them and to undergo a new ovarian stimulation cycle to increase the total number of oocytes as, according to our results, patients with <10 MII oocytes had reduced chances of obtaining at least 1 developing euploid blastocyst (unpublished data).
All patients were provided with specific information about the treatment and strategy proposed and signed a specific informed consent form.
The project was approved by the “Cátedra de Investigación en Obstetricia y Ginecología” of the Department of Obstetrics and Gynaecology, Hospital Universitari Dexeus, Universitat Autònoma de Barcelona.
Ovarian stimulation, vitrification and IVF
Ovarian stimulation protocols with GnRH analogues and gonadotropins were used as previously described21. Cryopreservation of MII oocytes was achieved with the vitrification technique using Cryotop® as described by Kuwayama2 and oocytes were stored in liquid nitrogen vapour.
Oocyte warming was performed on the same day as the oocyte retrieval of the last stimulation cycle8. All MII oocytes (fresh and warmed) were treated as a single cohort. Fresh MII oocytes and those which survived the warming process were microinjected 4 h post-retrieval and 2 h post-warming, respectively. Gamete and embryo handling was performed using fertilization and handling media from LifeGlobal®. Embryo culture was carried out in a tri-gas incubator with 5% O2 atmosphere using a single-step culture medium (Global, LifeGlobal®).
Embryo biopsy and genetic analysis
Embryos with ≥5 cells and <30% fragmentation at 62-68 hours post-ICSI were considered suitable for biopsy. Laser technology was used for drilling the zona pellucida22 and a single blastomere per embryo was aspirated. Whole-genome amplification and array-CGH analysis were performed using SurePlex®, 24Sure® V3, and BlueFuseMulti® (Illumina®) in accordance with the manufacturer’s instructions.
Anomalies were categorized as “simple aneuploidy” when affecting ≤2 chromosomes and as “complex aneuploidy” when affecting ≥3 chromosomes.
Transfer, vitrification and follow-up
Fresh transfer of euploid blastocysts was performed on D5 under ultrasound guidance23. Non-transferred euploid blastocysts were vitrified on D5 or D6 for further attempts using the kits and media from Kitazato® as previously described2.
Micronized vaginal progesterone was used for luteal phase support (200 mg every 8 hours) until plasma determination of beta-hCG ten days post-transfer. Clinical pregnancy was confirmed by the presence of a gestational sac with positive foetal heartbeat on ultrasound scan.
Statistical Analysis
Continuous variables were described by their mean and standard deviation. Frequency distributions were used to describe categorical or nominal variables.
Continuous variables were compared between groups with the Wilcoxon signed-rank test for paired samples.
Comparison between frequency groups was performed using the McNemar test for paired proportions.
All tests were bilateral, with a significance level of 5%.
Results
A total of 95 PGT-A patients underwent 120 oocyte vitrification cycles (X=1.3±0.5 cycles/patient) followed by a last cycle (fresh oocytes) for each patient. The mean age of the patients at the time of last oocyte pick-up (OPU) was 38.9±3.9 years. The antral follicle count of the included patients was 10.9±5.5 and the AMH level was 1.3±1.2 ng/ml. The median number of days elapsed between the first and the last OPU was 62 (43-345). A mean of 6.9±2.2 oocytes per patient were obtained and vitrified in the accumulation cycles. In the last treatment cycle (fresh cycle), 7.7±3.4 MII oocytes were obtained. Vitrified oocytes were warmed on the day of the last OPU and 81.6% survived. Warmed and fresh oocytes were microinjected as a single cohort. No differences were observed in the fertilisation rate when comparing warmed and fresh oocytes (73.8% vs. 75.2%). A better developmental capacity up to D3 was observed in embryos from fresh oocytes: 93.8% of the embryos from fresh oocytes were biopsied as opposed to 75.6% of the embryos from warmed oocytes (p<0.05). A mean of 8.9±3.1 D+3 embryos were biopsied per patient (3.1 from warmed oocytes + 5.8 from fresh oocytes). The euploidy rate was 16.9%, with no differences according to oocyte origin (18% in embryos from warmed oocytes vs. 16.2% in embryos from fresh oocytes). A simple abnormality was detected in 49.7% of the abnormal embryos, whereas 50.3% had complex abnormalities, with no differences found between embryos from warmed or fresh oocytes. The rate of euploid embryos reaching the blastocyst stage was not different between the two cohorts (72.2 % from warmed oocytes vs 81.0% from fresh oocytes). See Table 1.
The ratio of euploid blastocysts to MII oocytes in the vitrified/warmed group was 6.7% (44/659) and in the fresh group it was 10.3% (76/734). A 35.6% reduction in efficiency was observed in embryos derived from vitrified oocytes (Table 1).
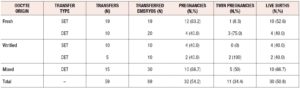
Overall, 89 embryos were transferred in 59 procedures (X=1.5±0.5 embryos transferred per patient). Fifty-four embryos were from fresh oocytes and 35 from previously vitrified oocytes. Thirty-six patients did not reach transfer (37.9%) due to the lack of euploid blastocysts. Forty-three embryos implanted in 32 women, representing an implantation rate of 48.3%. The clinical pregnancy rate was 33.7% per patient and 54.2% per transfer. The twin pregnancy rate was 34.4%. The ongoing pregnancy rate per patient was 32.7%, with one ectopic pregnancy and no miscarriages. There was an antepartum stillbirth at 31 weeks in a monochorionic monoamniotic twin pregnancy due to amniorrhexis. The remaining 30 pregnancies were full-term deliveries with 40 live births. The live birth rate per patient was 31.6% (Table 2).
In 54 of 59 transfer procedures it was possible to ascertain the origin of the implanted embryo (fresh or warmed), as these were homogeneous or heterogeneous transfers with a 0% or 100% implantation rate (KID-known implantation data). Analysis of the KID embryos did not show any significant difference in the implantation ability of the euploid embryos from warmed or fresh oocytes (43.3% and 47.9%).
Discussion
Our data allow analysis of the oocyte accumulation strategy used in PGT-A cycles to increase the number of available embryos. This strategy can be considered for patients with poor ovarian response to stimulation. Moreover, valuable data have been obtained regarding the effect of vitrification on embryo development in patients with a PGT-A indication.
The oocyte survival rate observed in our study is comparable to the rate described in other PGT-A groups24,25. However this rate is lower than that observed in our oocyte donation programme (81.6 vs 87.0%, Dexeus unpublished data) and reported in published data by other groups5,26. This can be attributed to the fact that donor oocytes constitute a good prognosis group with better oocyte quality (<35 years).
Our results showed the same fertilisation rate in fresh and warmed oocytes, in agreement with data reported by other authors4,6,8. The percentage of good-morphology embryos on D3, and therefore suitable for biopsy, was lower in the group of warmed oocytes than in the fresh oocyte group. Cellular damage in the oocyte during vitrification and warming could be at the origin of developmental impairment in the embryo. These results are in accordance with data from IVF patients, undergoing or not undergoing PGT-A, although the results are still controversial4, 6, 24, 27. The fact that the blastocyst rate after D3 biopsy is the same in both groups (warmed vs fresh) could indicate that the detrimental effects of vitrification are expressed mainly in the development of the embryo up to D3, which is consistent with the fact that the embryo relies on oocyte products before embryo genomic activation on D3.
Overall, our results agree with other data showing a reduction in the blastocyst rate of PGT-A embryos from vitrified oocytes24,25. However, data from a recently published paper20 did not show any reduction in the developmental ability of embryos derived from vitrified oocytes probably due to intrinsic characteristics of the patients in that particular study, as the patients included were younger (mean maternal age 36.6 vs 38.9) and the indications for PGT-A were different (PGT-A only for repeated implantation failure or recurrent miscarriage).
Our data do not show any differences in the rate or type of aneuploidy in embryos coming from fresh or warmed oocytes, endorsing previous findings20,24,25 and confirming that oocyte vitrification does not induce aneuploidy. Moreover, our data on KID outcomes indicate that the implantation ability of transferred euploid blastocysts may not be affected by the vitrification process, although this cannot be confirmed as implantation could not be followed up in all cases (KID from 54 out of 59 transfers). Notably, a mean of 1.5 embryos were replaced in each transfer, obtaining a multiple pregnancy rate of 34.4%. Although the current transfer policy in our programme is SET, by the time the study was performed, and due to the fact that fully expanded euploid blastocysts were not always available on D5, patients sometimes opted for double blastocyst transfer.
Before the present study, oocyte accumulation for PGT-A had already been used by other authors in advanced maternal age patients18 with good clinical outcomes. Our results confirm these data, although a reduction of 35% is observed in the performance of the vitrified oocytes when compared with the fresh ones, an aspect not assessed in the aforementioned study. This lower performance is due to the loss of oocytes during the warming process and the lower developmental ability up to D3 of embryos coming from vitrified oocytes.
As shown by our data, oocyte vitrification in patients eligible for PGT-A has a detrimental effect on the efficiency of the cycle, which suggests that the use of an oocyte accumulation strategy should be considered with caution in such patients. Alternative strategies would be either embryo accumulation (biopsied embryo vitrification) or the performance of consecutive fresh PGT-A cycles. Efficiency, cost-related and ethical issues, as well as emotional stress due to possible successive non-transfer cycles have to be assessed before deciding on the appropriate strategy for each patient.
In conclusion, oocyte accumulation in PGT-A patients makes it possible to increase the number of oocytes and, as a consequence, the embryos for analysis. However, in the era of trophectoderm biopsy and considering the excellent results after blastocyst vitrification, the loss of efficiency of vitrified oocytes in PGT-A patients discourages the use of this strategy. By comparing the performance of fresh and vitrified oocytes, we have been able to confirm that vitrification is a safe technique that does not induce aneuploidy; we also quantified the loss that oocyte vitrification implies for this group of women. Data obtained regarding the detrimental effect of vitrification on final embryo availability in patients with a PGT-A indication are also valuable for better patient counselling before the use of strategies involving oocyte vitrification.
Acknowledgements
This work was performed under the auspices of the Càtedra d’Investigació en Obstetrícia i Ginecologia of the Department of Obstetrics and Gynaecology, Hospital Universitari Dexeus, Universitat Autònoma de Barcelona.
Declaration of interests
Non declared.